We describe a case of primary meningococcal Y effusive pericarditis in a previously fit and well 35-year-old man who presented with a rapidly developing pericardial effusion resulting in cardiac tamponade. This is a rare, but important, cause of primary pericardial disease, and only the fourth documented case of primary meningococcal pericarditis due to Neisseria meningitidis serotype Y. Our patient was successfully treated with a pericardial drain and intravenous ceftriaxone. Our case highlights the importance of adverse clinical features such as temperature >38°C, subacute course, large effusion or tamponade, and non-steroidal anti-inflammatory drug (NSAID)/aspirin failure, which can identify patients who require close observation as they are at higher risk of complications.
Case
A 35-year-old man, with no past medical history, self-presented to the emergency department at 20:00 with sharp central chest pain across his sternum, worse on inspiration. This was associated with a temperature of 39.1°C and sweating, and had been preceded by a two-day history of viral head-cold symptoms. He had no history of foreign travel, headache, photophobia, or features suggestive of meningism. On examination he looked pale, but was comfortable and alert with a heart rate of 84 beats per minute and a blood pressure of 115/75 mmHg. Routine blood tests showed C-reactive protein (CRP) 39 mg/L, white blood cell count (WBC) 15.9 × 109/L. A 12-lead electrocardiogram (ECG) (figure 1) showed saddle-shaped ST elevation throughout the chest leads, consistent with pericarditis, and an echocardiogram showed preserved left ventricular systolic function and no pericardial effusion. He was, therefore, diagnosed with viral pericarditis and a chest infection, and was discharged at 04:30 the following morning with doxycycline and a plan to return to ambulatory care for repeat blood tests.
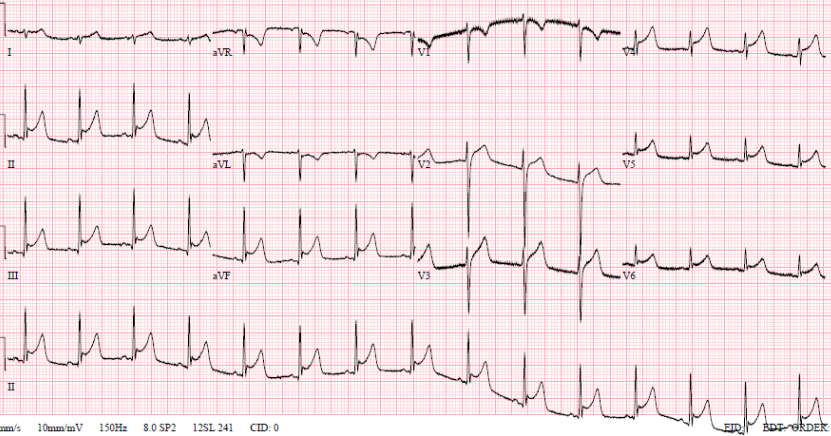
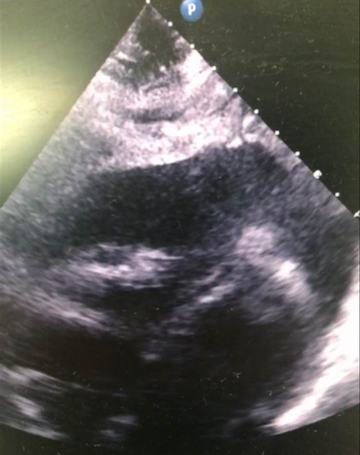
Later that night, an ambulance was urgently called as he was feeling more unwell, with dizziness and vomiting. The ambulance crew found him to be hypotensive with a blood pressure of 70/40 mmHg; improving to 90/50 mmHg with 1,100 ml of fluid by the time of his arrival to the emergency department. His heart sounds were quiet on examination. An ultrasound scan in the emergency department revealed a pericardial effusion. Initial point-of-care testing showed he had a metabolic acidosis with a venous pH of 7.27 and lactate 4.7 mmol/L. Emergency bedside echo showed cardiac tamponade with a 2.2–2.4 cm pericardial effusion globally, and right ventricular (RV) diastolic collapse (figure 2). He was taken to the catheterisation laboratory for emergency pericardiocentesis. He had an unrecordable blood pressure on arrival to the lab, and 300 ml of turbid yellow fluid was drained from the pericardium and sent for microscopy, culture and sensitivity (MC&S). Repeat echo showed <0.5 cm rim, and the patient improved haemodynamically. A pericardial drain was left in situ. Bloods showed his CRP had risen from 39 mg/L to 321 mg/L; with WBC increasing from 15.9 × 109/L to 22.1 × 109/L. Troponin T was now elevated to 117 ng/L compared with 13.68 ng/L on his first presentation. He was empirically started on co-amoxiclav 1.2 g intravenously.
Preliminary results from microbiology showed that he had grown Gram-negative diplococci on both blood cultures and pericardial fluid, with Gram stains awaited. Antibiotic therapy was changed to ceftriaxone 2 g intravenously twice daily. Subsequent results showed that he had grown Neisseria meningitidis in both blood cultures and cultures from the pericardial effusion. Blood cultures subsequently taken during his admission were negative after starting antibiotics.
His pericardial drain was removed after six days, after an echocardiogram had demonstrated limited residual effusion. Left ventricular (LV) and RV function remained normal on echocardiography, with no valvular abnormalities seen. His clinical course was generally stable and inflammatory markers improved with antibiotic therapy. He had no signs of meningitis at any point, and so no lumbar puncture or cerebral imaging was performed.
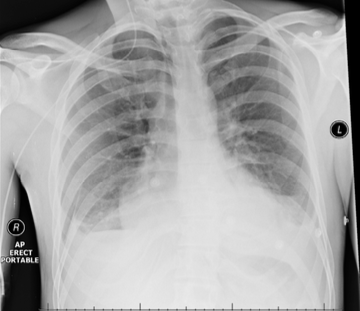
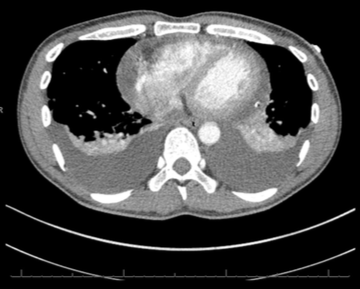
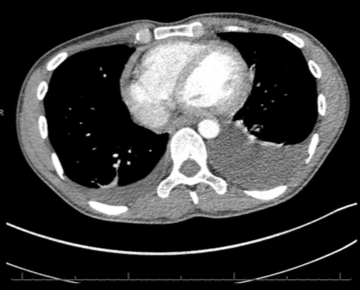
Throughout admission he was discussed multiple times by the heart team. Serial chest computed tomography (CT) scans showed bilateral pleural effusions, left side larger than the right, with some residual posterior pericardial collection (figures 3 and 4).
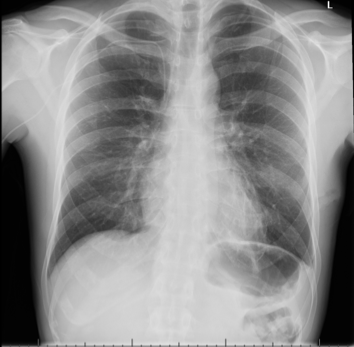
He, therefore, underwent a video-assisted thoracoscopic surgery (VATS) procedure 22 days after admission for this, with a left-sided chest drain: 300 ml of yellow fluid was drained initially, and 540 ml drained in total. The drain resulted in an apical pneumothorax, which was managed with underwater seal chest drain and suction. The drain was removed once the lung had re-expanded. The patient was asymptomatic from this (figures 5 and 6). Serial echocardiography showed improvement in pericardial effusions without the need for further drainage.
There was no evidence of pericardial thickening or calcification on CT scans performed in hospital, and magnetic resonance imaging (MRI) performed while an inpatient demonstrated normal cardiac function with normal appearances of the pericardium.
There was no growth in the pleural fluid and no pus cells seen; culture was negative for acid-fast bacilli.
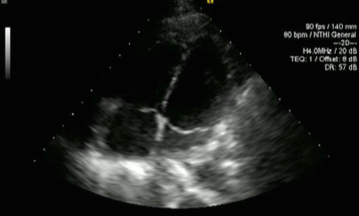
The patient was discharged with a further two weeks of intravenous antibiotics to give a total of six weeks’ antibiotic therapy. On review in clinic two weeks after discharge, his inflammatory markers showed CRP of 2 mg/L and WBC 8.2 × 109/L, with his latest imaging showing significant improvement in pleural and pericardial effusions. Echocardiography one month following admission showed resolution of the pericardial effusion (figure 7).
Discussion
Our case highlights an uncommon causative organism in acute pericarditis, demonstrating rapid deterioration and the need for emergency treatment.
Pericarditis is the most common pericardial disease worldwide, which has a variety of infectious and non-infectious causes. Of infectious causes, viruses, such as Coxsackie and Enteroviruses, account for 80–90% of cases.1,2 Non-tuberculous bacterial pericarditis accounts for fewer than 1% of cases, with meningococci accounting for only 5.9% of these, fourth behind streptococci, pneumococci and staphylococci.3 Pericarditis secondary to meningococci was first reported in 1918,4 and can be separated into three distinct groups, disseminated meningococcal disease (DMD), immune-reactive pericarditis (IRP) and primary meningococcal pericarditis (PMP).5
Primary meningococcal pericarditis is defined as purulent pericarditis caused by Neisseria meningitidis without clinical evidence of meningococcaemia, meningitis, or other foci of meningococcal infection.6
There are approximately 30 cases of PMP described in English literature, with the typical patient being a young male adult with an average age of 28 years (95% confidence interval [CI] 18 to 38).6 The most common presenting features are of chest pain, fever and shortness of breath, with usually a two-day delay until a cardiac cause is identified.3,6 With regards to investigations, previous reviews of the literature have found that leucocytosis is a common finding,3,6 but only 44% of cases were associated with positive blood cultures,6 and in a review by Blaser et al.3 93% of cases were associated with ECG abnormalities. Importantly, PMP is usually associated with the development of cardiac tamponade, with a review of 17 cases by Baevsky6 finding 15 of the patients went on to develop cardiac tamponade. In a similar finding to our case, Blaser et al.3 found that 10 of the 16 cases reviewed were complicated by sterile pleural effusions.
The human nasopharynx is the only known reservoir for Neisseria meningitidis, with roughly 5% of the population being asymptomatic long-term carriers, a figure that can rise to 90% during epidemic outbreaks.7 While serotypes B and C are still the most common identifiable causes of invasive meningococcal disease in Europe, their annual notification rate has fallen, while that of serotype Y has increased by 10.6% (95%CI 7.4 to 14.0) annually between 2004 and 2014.8 In previously documented cases of PMP, Neisseria meningitidis serotype C is the most common cause, followed by B and then W-135.6 There have been three previously documented cases of PMP due to meningococci serotype Y,9-11 with the 2008 case report by Zeidan et al. describing a similar case to ours in whom cardiac tamponade developed rapidly after initially presenting with presumed viral pericarditis.
The prognosis from appropriately treated PMP is favourable, with all case reports in English literature describing patients who have survived,6,9,12 compared with a fatality rate of 8.6% in patients with invasive meningococcal disease.10 However, difficulties in differentiating PMP from viral pericarditis does pose a problem, as patients are usually systemically well on presentation,9,11,12 yet identification and treatment of these patients before significant complications develop, such as cardiac tamponade, is key to a good outcome. A prospective cohort study of 453 patients, followed up for a mean period of 31 months, identified that specific clinical features (temperature >38°C, subacute course, large effusion or tamponade, and non-steroidal anti-inflammatory drug [NSAID]/aspirin failure) could potentially identify those patients where an identifiable cause could be isolated and are at higher risk for poor outcomes or complications.13 These criteria have been suggested as requiring inpatient management, our patient did have a fever of 39°C when initially seen in the emergency department, and so would have met these criteria.
Recent European Society of Cardiology (ESC) guidelines support these findings and recommend hospital admission for patients with any ‘high-risk features’ presenting with pericarditis,14 and given our patient was admitted with a fever of over 38°C, he would have met this criterion, even in the absence of a pericardial effusion at first admission.
Purulent pericarditis or the suspicion of a purulent effusion is an indication for pericardiocentesis, regardless of the presence or absence of tamponade, as untreated purulent pericarditis carries a grave prognosis.14 Due to the nature of the effusion in these cases, pericardial rinsing can also be considered to improve the complete drainage of material and help recovery. Intrapericardial administration of thrombolysis may help in cases of loculated effusions allowing more complete drainage and reducing the risk of long-term complications. With the risk of developing constrictive pericarditis following bacterial pericarditis being 20–30%, compared with <1% in viral/idiopathic pericarditis,15 these patients should be monitored in the outpatient setting for symptoms and signs that may suggest development of this complication. If there is any suspicion of constrictive pericarditis then the patient should be further assessed with transthoracic echocardiography, and, if constriction is identified, then they should be managed as per the recent ESC guidelines.14
Conclusion
We present an extremely rare case of primary meningococcal pericarditis caused by the Y-serotype form of the pathogen. The rapid course of deterioration in this patient mirrors that in published literature, and highlights the need for appropriate triage of patients presenting with suspected pericarditis. European guidelines advocate admission of patients with acute pericarditis in certain situations with high-risk features, and, as in our case, the development of tamponade due to pericarditis can be life-threatening. Treatment is multi-modal, with diagnostic and therapeutic drainage of effusions in conjunction with appropriate antimicrobial therapy. Care must be taken in surveillance to allow early identification of complications in the short and long term.
Key messages
- Primary meningococcal pericarditis is a rare, but serious, cause of pericarditis, often presenting with rapidly deteriorating clinical features in previously well patients
- Urgent recognition and treatment is essential, particularly when there are adverse clinical features apparent at presentation
- Treatment is via drainage of pericardial effusion, aggressive antibiotic therapy, and serial follow-up is essential
Conflicts of interest
None declared.
Consent
Permission to publish was obtained from the patient.
References
1. LeWinter M. Acute pericarditis. N Engl J Med 2014;371:2410–16. https://doi.org/10.1056/NEJMcp1404070
2. Imazio M, Spodick D, Brucato A, Trinchero R, Adler Y. Controversial issues in the management of pericardial diseases. Circulation 2010;121:916–28. https://doi.org/10.1161/CIRCULATIONAHA.108.844753
3. Blaser M, Reingold A, Alsever R, Hightower A. Primary meningococcal pericarditis: a disease of adults associated with serogroup C Neisseria meningitidis. Clin Infect Dis 1984;6:625–32. https://doi.org/10.1093/clinids/6.5.625
4. Herrick W. Meningococcal pericarditis, with report of 12 cases. Med Clin North Am 1918;2:411–26.
5. Finkelstein Y, Adler Y, Nussinovitch M, Varsano I, Amir J. A new classification for pericarditis associated with meningococcal infection. Eur J Pediatr 1997;156:585–8. https://doi.org/10.1007/s004310050669
6. Baevsky R. Primary meningococcal pericarditis. Clin Infect Dis 1999;29:213–15. https://doi.org/10.1086/520165
7. Javid M. Meningococcemia. Available at: https://emedicine.medscape.com/article/221473-overview#a4 [accessed 4 July 2018].
8. Whittaker R, Dias J, Ramliden M et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine 2017;35:2034–41. https://doi.org/10.1016/j.vaccine.2017.03.007
9. Zeidan A, Tariq S, Faltas B, Urban M, McGrody K. A case of primary meningococcal pericarditis caused by Neisseria meningitidis serotype Y with rapid evolution into cardiac tamponade. J Gen Intern Med 2008;23:1532–5. https://doi.org/10.1007/s11606-008-0685-y
10. Vienne P, Ducos-Galand M, Guiyoule A et al. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin Infect Dis 2003;37:1639–42. https://doi.org/10.1086/379719
11. Nkosi J, Thakrar A, Kumar K et al. Meningococcal serotype Y myopericarditis. Diagn Microbiol Infect Dis 2009;63:223–7. https://doi.org/10.1016/j.diagmicrobio.2008.09.015
12. Woudstra O, Boink G, Winkelman J, van Stralen R. A rare case of primary meningococcal myopericarditis in a 71-year-old male. Case Rep Cardiol 2016;2016:1297869. https://doi.org/10.1155/2016/1297869
13. Imazio M, Cecchi E, Demichelis B et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007;115:2739–44. https://doi.org/10.1161/CIRCULATIONAHA.106.662114
14. Adler Y, Charron P, Imazio M et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Eur Heart J 2015;36:2921–64. https://doi.org/10.1093/eurheartj/ehv318
15. Imazio M, Brucato A, Maestroni S et al. Risk of constrictive pericarditis after acute pericarditis. Circulation 2011;124:1270–5. https://doi.org/10.1161/CIRCULATIONAHA.111.018580
