Background, epidemiology and rationale for the COMPASS study
One quarter of all deaths in the UK in 2017 occurred as a result of diseases of the heart and circulation.1 One in seven men and one in twelve women died from coronary heart disease (CHD).1 The presence of CHD doubles the risk of stroke,2 and more than 100,000 strokes occur in the UK each year.1 Although the mortality rate from circulatory diseases is declining due to advances in treatment,1,3 more than 100,000 deaths resulted from CHD or stroke combined in the UK each year.1 CHD and stroke are the two leading causes of death worldwide.4 Circulatory disease is also associated with a heavy burden of morbidity. For example, more than 200,000 hospital visits in the UK were due to myocardial infarction (MI) each year in 2015–2017.5 CHD cost the UK economy approximately £19 billion per annum.6
Current guidelines recommend secondary prevention therapy with a single antiplatelet agent and a lipid-lowering agent for patients with established cardiovascular disease. Despite this, the annual recurrent event rate in patients on this combination of medications is still up to 5–10% per year.7 A meta-analysis demonstrated superior secondary prevention of adverse cardiovascular outcomes with the use of high-intensity oral anticoagulation (with a target for international normalised ratio [INR] of >2.8), or moderate-intensity oral anticoagulation (INR 2–3) plus aspirin, compared with aspirin alone.8 High-intensity, but not moderate-intensity oral coagulation, was associated with higher risk of bleeding, including intracranial bleeding.8 Conversely, low-intensity oral anticoagulation (INR <2.0) combined with aspirin did not confer any benefit over aspirin alone but still increased major bleeding episodes. Maintenance of an optimum INR appears to be the key for achieving an optimum balance between efficacy and safety with this approach.8 Hence, anticoagulation alone or in combination has not been recommended routinely as secondary prevention for decades.
The WOEST (What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting) trial was conducted in a patient population on long-term oral anticoagulation with warfarin, who underwent percutaneous coronary intervention (PCI).9 This trial compared ‘double therapy’ (addition of clopidogrel to the warfarin) with ‘triple therapy’ (addition of dual antiplatelet therapy [DAPT]). At one-year follow-up, double therapy reduced the risk of bleeding events, death, MI, stroke, and stent thrombosis. These findings suggest that clopidogrel plus warfarin may be equally effective as and safer than triple therapy in patients undergoing PCI. However, the study was limited by being non-blinded and underpowered for efficacy outcomes.
Rivaroxaban is a selective direct Factor Xa inhibitor used in the treatment and prevention of venous thromboembolism10–12 and for prevention of embolic stroke or systemic embolism in non-valvular atrial fibrillation.13 In the PIONEER AF-PCI (Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI) study, low-dose (15 mg once daily) and very-low-dose (2.5 mg twice daily) rivaroxaban was compared with warfarin plus antiplatelet therapy in patients with non-valvular atrial fibrillation (NVAF) post-PCI.14 This was a safety study with a primary safety end point of clinically significant bleeding.** There was a relative risk reduction of 39% in clinically significant bleeding with low-dose and very-low-dose rivaroxaban-based strategies when compared with warfarin-based triple therapy through 12 months (17.4% for combined rivaroxaban groups vs. 26.7% for vitamin K antagonist therapy, p<0.001). Based on the results with rivaroxaban 15 mg once daily, the SPC was updated for patients with NVAF who undergo PCI with stent placement.15
** A composite of Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding or bleeding requiring medical attention evaluated throughout the 12-month treatment period.
The ATLAS ACS 2-TIMI 51 (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome – Thrombolysis In Myocardial Infarction 51) trial demonstrated the efficacy of rivaroxaban 2.5 mg twice daily in patients with acute coronary syndrome (ACS).16 Treatment with rivaroxaban, in addition to single or dual antiplatelet therapy, was associated with a reduction in cardiovascular mortality and stent thrombosis, compared with placebo. There was an increase in major bleeding in ATLAS ACS 2-TIMI 51 with rivaroxaban 2.5 mg twice daily versus placebo, although no increase in fatal bleeding. This study proved that a very low dose (2.5 mg twice daily) of rivaroxaban combined with antiplatelet therapy is an effective treatment option in the management of patients with ACS.
The outcome of these studies has been favourable to the optimal combination of oral anticoagulation with an antiplatelet agent to reduce recurrent coronary events and cardiovascular mortality in patients with atherosclerotic cardiovascular disease. Pathophysiologically, it is logical to opt for combination therapy given the contribution of platelets, Factor X and thrombin in the formation of thrombus. Excess thrombin generation that persists beyond the acute phase plays an important role in recurrent thrombotic events.17 However, the combination of warfarin with antiplatelet therapy has not shown benefit in peripheral artery disease (PAD) and has led to major bleeding events, including intracranial bleeding.18
The COMPASS study
Design
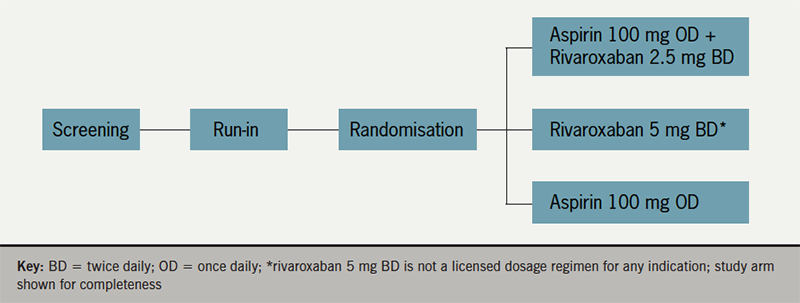
COMPASS (ClinicalTrials.gov NCT01776424) was a double-blind, double-dummy, randomised trial with a 3×2 partial factorial design, conducted at 602 centres in 33 countries and funded by Bayer.19 The trial involved four periods: screening, run-in period, follow-up and washout. The main objective of the trial (figure 1) was to evaluate whether rivaroxaban 2.5 mg twice daily in combination with aspirin 100 mg once daily, or rivaroxaban 5 mg once daily alone, reduced recurrent cardiovascular events, with acceptable safety, compared with aspirin 100 mg once daily, in patients with stable atherosclerotic vascular disease (CAD and/or PAD). Further details of the randomisation are given below.
A further comparison of pantoprazole versus placebo was conducted in the trial participants not receiving proton pump inhibitor (not shown in figure 1). Hence the study was a 3×2 factorial design. The steering committee comprising Population Health Research Institute (PHRI) investigators, study leaders in each country and representatives of the study sponsor, developed the protocol, which was approved by the relevant health authorities and institutional review boards. All patients provided written, informed consent. Data analyses were performed at PHRI.
Box 1 shows key inclusion and exclusion criteria for the study. Eligible patients entered a run-in phase up to 30 days, during which they received a rivaroxaban-matched placebo twice daily and aspirin 100 mg once-daily, designed to identify patients who develop an adverse event, or who were unwilling or unable to adhere or not suitable for randomisation. Post-coronary artery bypass graft (CABG) surgery patients were randomised 4–14 days after the surgery and did not enter the run-in.
Box 1. Key inclusion and exclusion criteria for the COMPASS trial
| Key inclusion criteria were: Coronary artery disease, difined as one or more of:
Peripheral artery disease, defined as one or more of:
Age 65 years, or age <65 years with documented atherosclerosis or revascularisation involving: at least two vascular beds or at least two additional risk factors (current smoking, diabetes mellitus, estimated glomerular filtration rate [eGFR <60 ml per minute, heart failure or non-lacunar ischaemic stroke ≥1 month earlier)
|
| Compiled from information presented in Eikelboom JW19 and ClinicalTrials.gov/ NCT01776424 |
Patients were randomised 1:1:1 ratio to receive rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg once daily), rivaroxaban (5 mg twice daily) with an aspirin-matched placebo once daily, or aspirin (100 mg once daily) with a rivaroxaban-matched placebo twice daily, stratified according to centre and the use of proton-pump inhibitor therapy at the time of randomisation. Study aspirin was enteric-coated. Patients who were eligible for the proton-pump inhibitor randomisation were also randomly assigned in a 1:1 ratio to receive pantoprazole (40 mg once daily) or matched placebo. After randomisation, participants were seen at one and six months and then at six-month intervals.
Primary and secondary outcomes
The primary efficacy outcomes were the composite of cardiovascular death, stroke, or MI. The primary safety outcome was a modification of the International Society on Thrombosis and Haemostasis (ISTH) criteria for major bleeding and included fatal bleeding, symptomatic bleeding into a critical organ, bleeding into a surgical site requiring reoperation, and bleeding that led to hospitalisation (including presentation to an acute care facility without an overnight stay). However, the study considered as a major bleed any bleeding that led to acute presentation or hospitalisation.
The secondary efficacy outcomes were composites of ischaemic stroke, MI, acute limb ischaemia, or death from CHD, and of ischaemic stroke, MI, acute limb ischaemia, cardiovascular death, and death from any cause. Tertiary efficacy outcomes included individual components of the primary and secondary outcomes, as well as hospitalisation for cardiovascular causes, revascularisation, limb amputation, stent thrombosis, angina, heart failure, venous thromboembolism, resuscitated cardiac arrest, and a new diagnosis of cancer. The net-clinical-benefit outcome was the composite of cardiovascular death, stroke, MI, fatal bleeding, or symptomatic bleeding into a critical organ. The main outcome for the pantoprazole versus placebo randomisation was upper gastrointestinal complications.
COMPASS was an event-driven trial, with an expected control event rate of 3.3/100 person-years; it was designed to continue until at least 2,200 participants had a confirmed primary efficacy outcome, thereby providing 90% power to detect a 20% lower risk in each of the two comparisons of rivaroxaban versus aspirin.
Results of the COMPASS trial
A total of 27,395 patients who successfully completed the run-in phase or who were enrolled after CABG surgery were randomised; 2,320 participants did not complete the run-in phase and were excluded. The study period was three to four years. Baseline characteristics were similar in all three arms. The mean age was 68.2 years, 90.6% had a history of CAD, and 27.3% of PAD. Lipid-lowering agents were used by 89.8%.
The independent Data and Safety Monitoring Board recommended early termination of the randomised comparison of rivaroxaban with or without aspirin versus aspirin alone at the first formal interim analysis for efficacy (50% of planned events). This was due to observation of a consistent difference in the primary efficacy outcome in favour of rivaroxaban plus aspirin (z = −4.592). The z statistic for the comparison of rivaroxaban plus aspirin versus aspirin alone was larger than the prespecified 4 standard deviations.
Figure 2 summarises some key results from the trial.19,20 Primary outcome events occurred in 379 patients (4.1%) in rivaroxaban plus aspirin group, 448 (4.9%) in rivaroxaban alone group, and 496 (5.4%) in aspirin alone group. For the comparison of rivaroxaban (2.5 mg twice daily) plus aspirin with aspirin alone, the hazard ratio for the primary outcome was 0.76 (95% confidence interval [CI], 0.66 to 0.86; p<0.001). For the comparison of rivaroxaban (5 mg twice daily) alone with aspirin alone, the hazard ratio (HR) was 0.90 (95% CI, 0.79 to 1.03; p=0.12).

The first secondary composite outcome occurred in 329 patients (3.6%) in the rivaroxaban-aspirin group and in 450 patients (4.9%) in the aspirin-alone group (HR 0.72; 95%CI, 0.63 to 0.83; p<0.001). The additional secondary composite outcome occurred in fewer patients in the rivaroxaban-plus-aspirin group than in the aspirin-alone group (389 patients [4.3%] vs. 516 patients [5.7%], HR 0.74 [95%CI, 0.65 to 0.85]; p<0.001). There were 313 deaths (3.4%) in the rivaroxaban-aspirin group compared with 378 (4.1%) in the aspirin-alone group (HR 0.82; 95% CI, 0.71 to 0.96; p=0.01). Formal testing of the secondary outcomes was not performed for rivaroxaban alone compared with aspirin alone because no significant effect was seen for the primary composite outcome.
More major bleeding events occurred in the rivaroxaban-plus-aspirin group than in the aspirin-alone group (288 patients [3.1%] vs. 170 patients [1.9%], HR 1.70 [95%CI 1.40 to 2.05]; p<0.001). Patients in the rivaroxaban-alone group also had more major bleeding events than in the aspirin alone group (255 patients [2.8%] vs.170 patients [1.9%], HR 1.51; [95%CI, 1.25 to 1.84]; p<0.001). Serious adverse events occurred in 721 patients (7.9%) assigned to rivaroxaban plus aspirin, 702 (7.7%) assigned to rivaroxaban alone, and 662 (7.3%) assigned to aspirin alone.
The composite net-clinical-benefit outcome of cardiovascular death, stroke, MI, fatal bleeding, or symptomatic bleeding into a critical organ was lower with rivaroxaban plus aspirin than with aspirin alone (431 patients [4.7%] vs. 534 patients [5.9%], HR 0.80 [95% CI, 0.70 to 0.91]; p<0.001).
A total of 7,470 patients had stable PAD or carotid artery disease, including 55% with symptomatic PAD of the lower extremities, 20% with asymptomatic PAD plus coronary disease, and 26% with prior carotid revascularisation or asymptomatic 50% carotid stenosis.20 In this PAD/carotid disease cohort, there were significant reductions for rivaroxaban-aspirin versus aspirin alone for the main primary and secondary outcomes (figure 2).
Implications of COMPASS trial results
The results of the trial are encouraging for patients with stable CAD and PAD in sinus rhythm. The primary outcome was 24% lower with rivaroxaban 2.5 mg twice daily plus aspirin than with aspirin alone (4.1% vs. 5.4%). But, the rate of major bleeding was 70% higher in the rivaroxaban-aspirin group (3.1% vs. 1.9%), predominantly accounted for by gastrointestinal bleeding. The rate of the net-clinical-benefit outcome was lower by 20% with rivaroxaban plus aspirin than with aspirin alone (4.7% vs. 5.9%).
Ticagrelor, one of the newer antiplatelet agents, was beneficial in reducing recurrent events when continued for one to three years at a low dose post-MI.21 However, patients with PAD did not demonstrate this benefit when compared with clopidogrel in the EUCLID (Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease) trial.22 Aspirin has shown to reduce major vascular events by 20% in secondary prevention trials in PAD patients.23 But efficacy data for aspirin monotherapy are lacking.24 The use of oral anticoagulation with antiplatelet agents in PAD is not supported by evidence of efficacy and, hence, no previous guidelines recommended anticoagulation in patients with PAD.
COMPASS is the first direct oral antagonist anticoagulant (DOAC) and antiplatelet combination therapy trial to demonstrate significant reduction in MACE in patients with stable atherosclerotic disease. Obviously, there is a trade-off with increased bleeding risk and the cost, which need to be taken into account. The UK National Institute for Health and Care Excellence (NICE) have recently published their technology appraisal guidance (TA607): ‘Rivaroxaban for preventing atherothrombotic events in people with coronary or peripheral artery disease’.25 In this document, NICE recommended rivaroxaban plus aspirin as an option for preventing atherothrombotic events in adults with CAD or symptomatic PAD who are at high risk of ischaemic events.
NICE defines a high risk of ischaemic events for people with CAD as:
- aged 65 years or over
- or with atherosclerosis in at least two vascular territories (such as coronary, cerebrovascular, or peripheral arteries)
- or two or more of the following risk factors: current smoking, diabetes, kidney dysfunction with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min (note that rivaroxaban is contraindicated if the eGFR is less than 15 ml/min), heart failure, previous non-lacunar ischaemic stroke.
NICE emphasised that a person’s risk of bleeding should be assessed before considering rivaroxaban. Treatment should only be started after an informed discussion with them about the risks and benefits of rivaroxaban, weighing up the risk of atherothrombotic events against the risk of bleeding. The risks and benefits of continuing treatment with rivaroxaban should be regularly reviewed.
Patients with a history of MI in COMPASS (about 62%) were, on average, over seven years post the acute event. The observed treatment benefit was consistent both in patients with more recent events and in those with events occurring many years previously.
The COMPASS trial has provided a new way of reducing the risk of MACE in patients with stable atherosclerotic disease who meet the above NICE recommendation criteria for treatment. Given that most of the patients with stable disease are looked after by primary care and are many years post-diagnosis, the majority of the prescriptions are likely to be made in the community.
Key messages
- Rivaroxaban combined with aspirin has demonstrated clinical benefit in patients with coronary artery disease
- The COMPASS trial showed that a combination of low-dose rivaroxaban and aspirin reduced the risk of adverse cardiovascular events in patients with stable atherosclerotic disease
- The results of the COMPASS trial were assessed by the UK National Institute for Health and Care Excellence:‘rivaroxaban plus aspirin is recommended within its marketing authorisation, as an option for preventing atherothrombotic events in adults with coronary artery disease or symptomatic peripheral artery disease who are at high risk of ischaemic events’
- Management guidelines in this area require updating to incorporate the results of COMPASS into routine clinical practice
Conflicts of interest
The authors participated in the COMPASS trial.
DC has received research funding, speaker fees and advisory consultation fees from Bayer.
Funding
The study was funded by Bayer.
Study approval
The multicentre study was approved by the relevant health authorities and institutional review boards.
Consent
All patients provided written, informed consent.
Subramanya G N Upadhyaya
Interventional Fellow
Vinoda Sharma
Consultant Interventional Cardiologist
Derek Connolly
Consultant Cardiologist and Honorary Senior Lecturer (Institute of Cardiovascular Science, University of Birmingham)
Email: (Derek.Connolly1@nhs.net)
Birmingham City Hospital, Sandwell and West Birmingham Hospitals NHS Trust, Dudley Road, Birmingham, B18 7QH.
Articles in this supplement
Introduction
Atherosclerotic peripheral artery disease: the growing challenge to improve life and limb
Peripheral artery disease: current diagnosis and management
Combining rivaroxaban with aspirin in stable atherosclerotic vascular disease: clinical evidence from the COMPASS study
![]() Once you have read all these articles, you can take the ‘Learning with reflection’ CPD activity on this supplement.
Once you have read all these articles, you can take the ‘Learning with reflection’ CPD activity on this supplement.
References
1. British Heart Foundation. CVD statistics – BHF UK Factsheet (2017). Available at https://www.bhf.org.uk/what-we-do/our-research/heart-statistics (last accessed February 2019).
2. Tran J, Norton R, Conrad N et al. Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study. PLoS Med 2018;15:e1002513. https://doi.org/10.1371/journal.pmed.1002513
3. Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016;102:1945–52. https://doi.org/10.1136/heartjnl-2016-309573
4. World Health Organization. The top ten causes of death (2018). Available at http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (last accessed February 2019).
5. UK hospital episode statistics, 2015/16/17; NHS Digital/ISD Scotland/NHS Wales/DH Northern Ireland
6. European Heart Network. Analysis of European cardiovascular disease statistics 2017, EHN. Available at www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html (last accessed February 2019).
7. Bhatt DL, Eagle KA, Ohman EM et al. Comparative determinants of 4-year cardio- vascular event rates in stable outpatients at risk of or with atherothrombosis.JAMA 2010;304:1350–7. https://doi.org/10.1001/jama.2010.1322
8. Anand SS, Yusuf S. Oral anticoagulants in patients with coronary artery disease. J Am Coll Cardiol 2003;41(suppl S):62S–69S. https://doi.org/10.1016/S0735-1097(02)02776-6
9. Dewilde WJ, Oirbans T, Verheugt FW et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–15. https://doi.org/10.1016/S0140-6736(12)62177-1
10. Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty: pooled analysis of four studies. Thromb Haemost 2011;105:444–53. https://doi.org/10.1160/TH10-09-0601
11. The EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. https://doi.org/10.1056/NEJMoa1113572
12. The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thrombo- embolism. N Engl J Med 2010;363:2499-510. https://doi.org/10.1056/NEJMoa1007903
13. Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. https://doi.org/10.1056/NEJMoa1009638
14. Gibson CM, Mehran R, Bode C et al. Prevention of bleeding in patients with AF undergoing PCI. New Engl J Med 2016;375:2423–34. https://doi.org/10.1056/NEJMoa1611594
15. Xarelto (rivaroxaban) Summary of Product Characteristics as approved by the European Commission.
16. Mega JL, Braunwald E, Wiviott SD et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9-19. https://doi.org/10.1056/NEJMoa1112277
17. Merlini PA, Bauer KA, Oltrona L et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994;90:61-68
18. The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anti-coagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med 2007;357:217–27. https://doi.org/10.1056/NEJMoa065959
19. Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. https://doi.org/10.1056/NEJMoa1709118
20. Anand SS, Bosch J, Eikelboom JW et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet (advance publication online). https://doi.org/10.1016/S0140-6736(17)32409-1
21. Bonaca MP, Bhatt DL, Cohen M et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. https://doi.org/10.1056/NEJMoa1500857
22. Hiatt WR, Fowkes FGR, Heizer G et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32-40. https://doi.org/10.1056/NEJMoa1611688
23. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. https://doi.org/10.1016/S0140-6736(09)60503-1
24. Brass EP, Hiatt WR. Aspirin monotherapy should not be recommended for cardioprotection in patients with symptomatic peripheral artery disease. Circulation 2017;136:785-6. https://doi.org/10.1161/CIRCULATIONAHA.117.028888
25. National Institute for Health and Care Excellence (NICE). Rivaroxaban for preventing atherothrombotic events in people with coronary or peripheral artery disease. Technology appraisal guidance (TA607). London: NICE, October 2019. www.nice.org.uk/guidance/ta607 (last accessed December 2019)
Notes on dosing recommendations from Xarelto® ▼ (rivaroxaban) SmPC (Summary of Product Characteristics)
Xarelto 2.5 mg twice daily, coadministered with a daily dose of 75–100 mg aspirin, is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial discussed in this supplement compared both Xarelto 2.5 mg twice-daily plus aspirin and also Xarelto 5 mg twice-daily without aspirin, versus aspirin alone. Results for both comparisons are provided reflecting the original study publication.
Please note, however, that Xarelto 5 mg twice-daily is not a licensed dosage regimen for the above, nor for any other therapeutic indication.