Introduction
|
The hidden epidemic of HF
Approximately 10–20% of patients with a diagnosis of HF at discharge from hospital initially receive treatment for something else, such as chronic obstructive pulmonary disease (COPD).1,2 As many as 16% of patients over the age of 65 presenting with breathlessness to their general practitioner (GP) may have HF as the cause.3 |
Heart failure (HF) is a syndrome and not a diagnosis. Once a patient has been identified as having the HF syndrome, a cause must be sought. For example, in the majority of patients with HF and a reduced ejection fraction (HFrEF), the underlying cause will be ischaemic heart disease (IHD).
The signs and symptoms of HF are common and non-specific; misdiagnosis and under-diagnosis are common (figure 1–3).
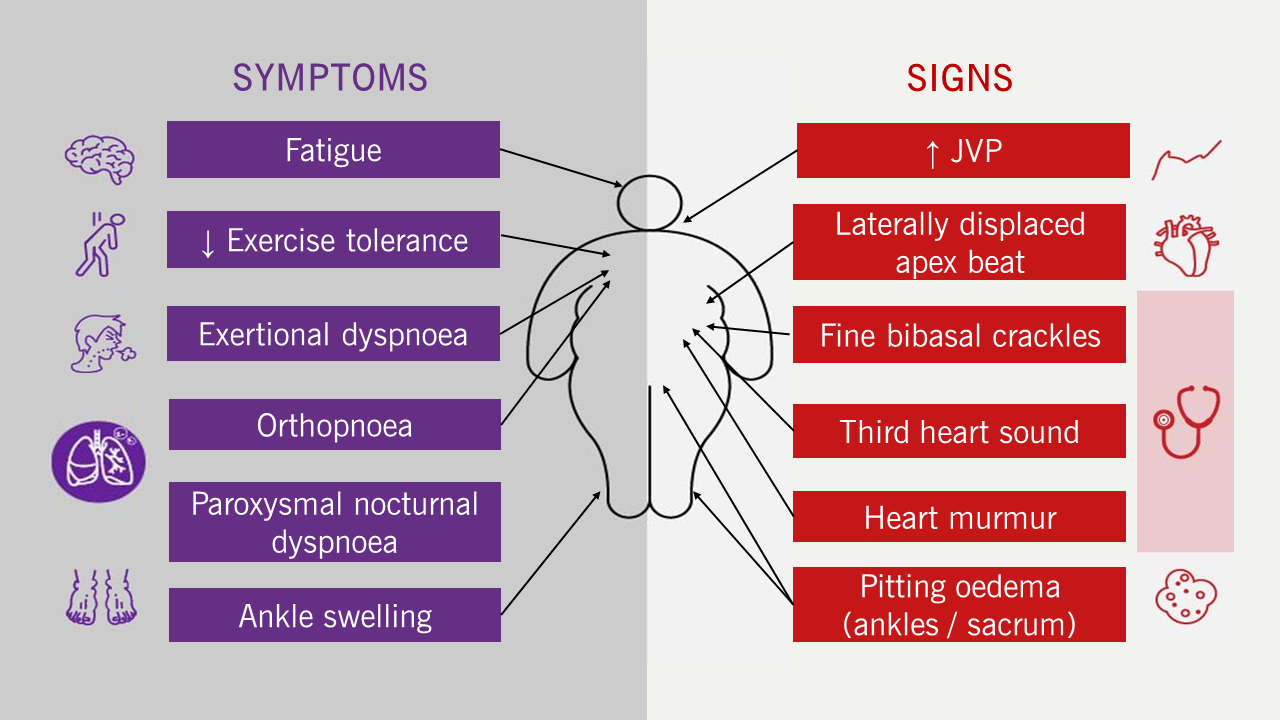
| Key: JVP = jugular venous pressure |
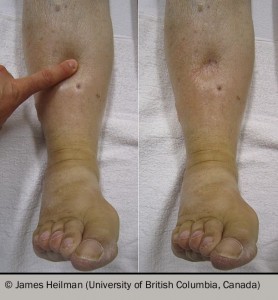 |
Figure 3. Jugular venous pressure (Click arrow below to play, or bottom-right for full screen)
|
Clinical presentation
|
||||||||
|
Table 1. New York Heart Association classification
|
A patient can be said to have the HF syndrome if they meet all of the following criteria:
- Typical symptoms and signs (figure 1)
- Evidence of cardiac dysfunction – raised serum natriuretic peptide (NP) concentration
- Structural and / or functional abnormalities of the heart detected on imaging
This module will cover the common investigations and tests used to diagnose and monitor patients with HF with specific focus on the National Institute of Health and Care Excellence (NICE) guidelines and the European Society of Cardiology (ESC) 2021 guidelines and the 2023 update.5–7
Symptom severity assessment
Assessing functional and exercise capacity is an important part of the clinical assessment of patients with HF. The New York Heart Association (NYHA) classification is a widely used tool to grade the severity of a patient’s symptoms (table 1). However, reproducibility of NYHA class is low, with low validity and reproducibility.8–10
Modes of presentation (table 2)
Acute or decompensated HF
If a patient with chronic HF deteriorates, either suddenly or slowly, the episode may be described as ‘decompensated’ HF which can be divided in to two modes of presentation. Additionally, a patient can also present acutely in cardiogenic shock, with or without a previous diagnosis of HF.
- Acute pulmonary oedema
A medical emergency usually triggered by an acute event, such as ischaemia or arrhythmia. - Anasarca
Anasarca refers to patients presenting with worsening symptoms or signs of fluid retention. Such patients are often presenting for the first time, but may be patients having an exacerbation of their previously stable HF. - Cardiogenic shock
Cardiogenic shock is multi-organ dysfunction due to hypoperfusion caused by a sudden fall in cardiac output. It is typically seen following a large (acute) myocardial infarction. It is a distinct clinical entity not further covered here.
Table 2. Differences between acute modes of presentation with heart failure
| Anasarca | Acute pulmonary oedema | Cardiogenic shock | |
| Symptoms | Comfortable at rest but breathless on minimal exertion | Pale and clammy Unable to lie flat or talk in full sentences |
Agitation, confusion, collapse |
| Signs and symptoms of precipitating cause, such as myocardial ischaemia or arrhythmia | Signs and symptoms of precipitating cause, such as myocardial ischaemia | ||
| Signs | Tachycardia – sinus tachycardia or atrial fibrillation | Tachypnoea, hypoxia, respiratory failure | Tachypnoea, hypoxia, tachycardia, hypotension |
| Low systemic blood pressure | Tachycardia | Cold peripheries Clammy |
|
| Pitting oedema (figure 2) †; raised JVP (figure 3); lung crackles | Pink, frothy sputum – oedema | Usually peri-arrest requiring urgent assessment and intervention | |
| Pleural effusion, ascites | Hypertension due to sympathetic activation | ||
| Key: AF = atrial fibrillation; JVP = jugular venous pressure † Oedema accumulates with gravity; in an ambulatory patient, the ankles are affected first rising to the knees, thighs and then sacrum sequentially. In bedbound patients, the sacrum is often affected first. Extreme fluid retention causes pleural effusions, ascites, pericardial effusions and abdominal or thoracic wall oedema. |
|||
Chronic HF
Patients with chronic HF have been treated medically and will usually have few, if any, symptoms or signs at rest. The term ‘congestive’ HF, often used to describe patients in this condition (particularly in North America), is inappropriate – patients with treated HF should not be congested.3,11
Investigations
Blood tests
NPs
|
Serum NP levels can be measured by immunoassay which is quick, easy and cheap. The major role for NP testing is in excluding the diagnosis of HF in a breathless patient; those patients with levels below defined cut-offs do not have HF and an alternative diagnosis for any symptoms should be sought. |
- NP release and actions
NPs are secreted by the myocardium in response to stretch caused by volume and/or pressure overload. In health, they are part of the homeostatic system which maintains blood volume. They counter-act many of the pathophysiological processes of HF (figure 4). - Measuring NPs in practice12
Low serum NP concentration has a high negative predictive value (94%) for the presence of HF. Serum NP concentration gives useful prognostic information: the higher the level, the worse the prognosis, regardless of underlying pathology.However, raised NP concentrations are not synonymous with HF. There are many causes of raised NPs (table 3). Very high NP levels ought to prompt investigations for other serious illness such as malignancy or bacteraemia. As with any test, the results of NP testing must be interpreted in the clinical context.
- B-type NP (BNP) or N-terminal pro-BNP (NT-proBNP)
- A-type NP (ANP)
- B-type NP (BNP)
- C-type NP (CNP) and
- urodilatin.
- A level greater than 2,000 ng/L should lead to referral for urgent specialist assessment with echocardiography within two weeks
- A level between 400–2,000 ng/L should lead to referral for specialist assessment with echocardiography within six weeks
- A level less than 400 ng/L makes HF unlikely and another cause for any symptoms should be sought.
- NP testing for patients admitted to hospital with HF
The NICE guidelines for acute HF recommend an NT-proBNP cut-off of <300 ng/L to rule out a diagnosis of HF in patients admitted to hospital.13 This is an unhelpful recommendation. Many conditions that may present as an emergency and mimic HF, including respiratory tract infections, anaemia, arrhythmia, old age and frailty, are also associated with raised NPs. It would be highly unusual for a patient presenting with pneumonia or atrial fibrillation (AF) with a fast ventricular rate to have a serum NT-proBNP concentration <300 ng/L.The likelihood of HF should always be based on the history and examination, and not on blood tests considered in isolation.
The cut off of 300 ng/L in acute HF is lower than that used for patients presenting in the community. The distinction leads to odd conclusions. For example, a patient presenting to their GP with signs and symptoms of HF and an NT-proBNP concentration of 350 ng/L has HF ruled out as a cause of their symptoms. However, if that same patient had presented to Accident and Emergency, HF would still be a possible diagnosis.
- NP testing for monitoring patients with confirmed HF
- Serum NPs are, at present, the best single prognostic test for patients with confirmed HF but serial measurements are not recommended for monitoring5
- NP concentrations are more closely related to outcome than left ventricular ejection fraction (LVEF) or other neurohormones in patients with advanced HF
- High serum NP concentrations are associated with an increased risk of sudden death in patients with chronic HF
- High NP concentrations are associated with an increased risk of in-hospital mortality, regardless of ejection fraction (EF)
- High NP concentrations at discharge are associated with increased risk of re-admission or death for at least six months post-admission
- An increase in NP concentration during admission is associated with increased risk of adverse events, whereas a decrease in serum NP is associated with lower risk.
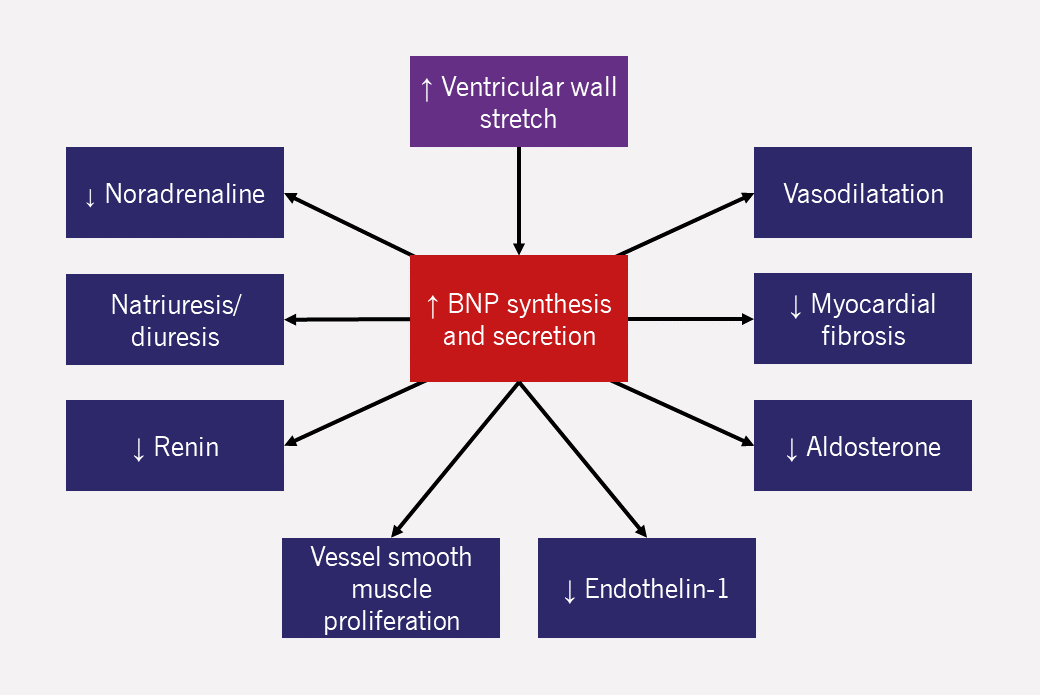
Table 3. Cardiac and non-cardiac causes of raised and reduced BNP and NT-proBNP
| Causes of raised NP levels |
|---|
| Cardiac |
| Right ventricular strain |
| Acute coronary syndrome |
| Heart muscle disease including LVH |
| Valvular heart disease |
| Pericardial disease |
| Atrial fibrillation |
| Myocarditis |
| Cardiac surgery |
| Cardioversion |
| Non-cardiac |
| Advancing age (>70) |
| Anemia |
| Renal failure |
| Pulmonary: OSA, pneumonia, pulmonary hypertension, PE, hypoxia, COPD |
| Sepsis |
| Burns |
| Liver failure |
| Diabetes |
| Causes of reduced NP levels |
| BMI >35 kg/m2 |
| Drugs, including diuretics, ACE inhibitors, ARBs, beta blockers, MRAs |
| African-Caribbean family origin |
| Key: ACE = angiotensin-converting enzyme; ARBs = angiotensin receptor blockers; BMI = body mass index; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; LVH = left ventricular hypertrophy; MRA = mineralocorticoid receptor antagonist; NP = natriuretic peptide; NT-proBNP = N-terminal fragment of the prohormone BNP; OSA = obstructive sleep apnoea; PE = pulmonary embolism |
There are four different NPs:
BNP is produced by cleavage of a precursor molecule, proBNP, into an inactive fragment (amino-terminal proBNP, NT-proBNP), and BNP itself. Both can potentially be measured (as can many other elements of the NP system). NT-proBNP is more stable and has a longer half-life than BNP and as a consequence, is the test recommended by NICE.
If the patient has symptoms and signs of HF with a raised NT-proBNP, the next step is specialist referral for cardiac imaging.5 NICE guidance for chronic HF gives detailed guidance on thresholds of NT-proBNP concentrations to guide referral.5
All patients with suspected HF should have NT-proBNP tested:5
The ESC HF guidelines 2021 have lower serum NP thresholds for referral for echocardiography: BNP >35 pg/ml and NT-proBNP >125 pg/ml.6
Other tests
Following diagnosis, other laboratory investigations should be performed for a patient with incident HF, including full blood count, urea and electrolytes, thyroid function, fasting glucose and glycosylated haemaglobin (HbA1c), lipids, and iron status.5,6 The aim is to detect common co-morbidities or treatable precipitants of decompensated HF. Baseline measurements of renal function and serum electrolytes also guide treatment. Upon an initial presentation of HF, one may wish to consider investigating for underlying amyloidosis, which includes urine and serum protein electrophoresis. (See figure 18 and tables 4 and 5 below)
The electrocardiogram
All patients with suspected HF or decompensated HF should have a 12-lead electrocardiogram (ECG) as part of their investigations. It may be diagnostic and guide future management. It is also helpful to compare the latest ECG to older ones to detect any changes. For example:
- Q-waves may indicate an established area of infarcted myocardium giving a clue to the aetiology;
- increased voltages in the leads over the left ventricle (LV) (V3–V6) suggest LV hypertrophy, perhaps due to hypertensive or genetic cardiomyopathy;
- approximately a quarter of patients with HF have AF at presentation, which should be managed with anticoagulation and appropriate rate or rhythm control:
- in some patients, AF with an abnormally fast (or slow) ventricular rate may be the cause of HF;
- high degrees of atrioventricular (AV) block causing bradycardia is a curable cause of HF.
A normal ECG has a high negative predictive value (93%) for excluding LV systolic dysfunction.15
In chronic HF, serial ECGs may detect incident AF or incident left bundle branch block (~10% per year),16,17 which might change management, for example, with cardiac resynchronisation therapy (module 4).
Functional capacity assessment
|
Normal exercise capacity in a patient not receiving treatment effectively excludes the diagnosis of symptomatic HF. However, there is a poor correlation between exercise capacity and resting haemodynamic measures, including EF. |
Objective measures of functional and exercise capacity, such as the six-minute walk test (6MWT) or incremental exercise test, give more reliable information regarding a patient’s exercise capacity and may be useful to diagnose and assess the severity of HF. However, both are susceptible to a ‘learning effect’ and can be difficult to use routinely in a busy clinic.
They include the following:
- The 6MWT
- Incremental exercise capacity tests21
- Gas exchange analysis using the peak oxygen consumption (peak VO2)
The 6MWT measures the distance walked during six minutes on a hard, flat surface. The patient goes at their own pace and rests as needed. It can be performed in the corridor of a ward or outpatient clinic without the need for equipment; the recommended corridor length is 30 metres.18
In healthy adults, the normal 6MWT distance is between 400–700 metres. In patients with HF, a 6MWT distance less than 300 metres is associated with worse cardiovascular outcomes.19,20
While the 6MWT is a test of aerobic endurance, incremental exercise tests, such as the Bruce treadmill test or incremental shuttle walk test, are a better indicator of maximal aerobic performance. In patients without significant limitation, incremental exercise tests may be preferable to the 6MWT for assessing the exercise capacity of patients with HF.
An incremental protocol can be coupled to the measurement of metabolic gas exchange to derive objective measures of exercise capacity, such as the anaerobic threshold, peak VO2 and the ventilatory response to exercise. Such tests can be helpful in differentiating dyspnoea of cardiac or respiratory origin, and are often used as part of the assessment for heart transplantation.
Haemodynamic studies22–25
Haemodynamic assessment of patients with HF can give information on a patient’s fluid status. They are invasive, with uncertain clinical benefit in the majority of patients. Continuous haemodynamic monitoring in ambulatory patients is possible via an implantable device but these are not routinely available.22
Implantable devices for continuous measurement of pulmonary artery pressure (and even left atrial pressure) have been developed. Pulmonary artery pressure increases in patients with chronic stable HF as they decompensate, often before the onset of symptoms.23–25 Some studies have shown that an implantable device that measures pulmonary artery pressure results in patients receiving more aggressive medical therapy and reduces hospital admissions with HF.23–25 However, the device carries procedural risk and the data generated requires the time and resources of already overstretched healthcare professionals to be put to use. As a result, such devices are far from widespread adoption.
Measuring cardiac output with thermodilution together with direct measurement of intracardiac pressures can occasionally be useful in patients hospitalised with severe or refractory HF.22 Right heart catheterisation is a key investigation in assessing suitability for heart.22
Imaging
Chest radiograph/X-ray
|
|
|
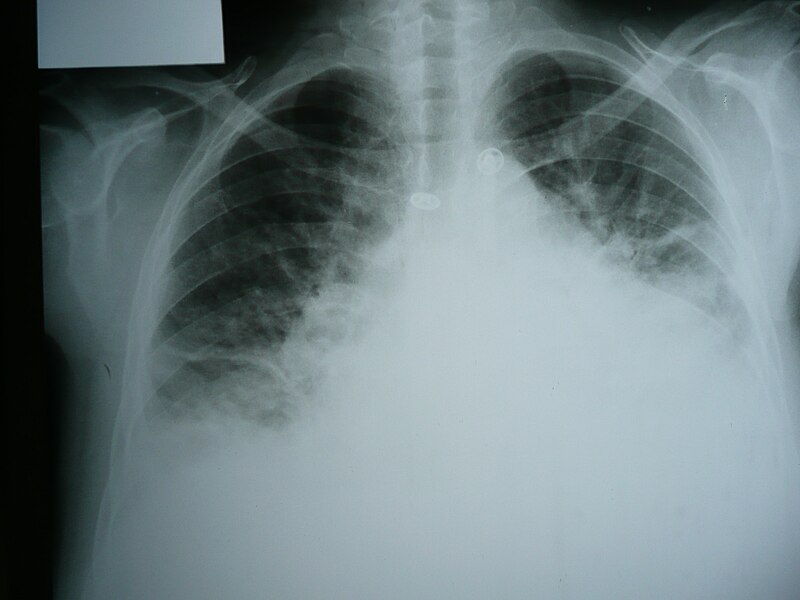
A chest X-ray (CXR) (figure 5) is an essential investigation for anyone presenting with breathlessness, regardless of whether the patient is known to have HF or lung disease. In patients with acute HF, it may show:5
- alveolar shadowing indicative of frank pulmonary oedema – fluffy opacities throughout the lung fields
- upper lobe blood diversion in the pulmonary vasculature – as blood is diverted to the upper lobes to compensate for the ventilation-perfusion mismatch caused by pulmonary oedema
- Kerley B lines – interstitial oedema appearing as short horizontal lines in the peripheries of the lung field
- pleural effusion
- cardiomegaly – may be the only abnormality in patients with chronic HF.*
* Cardiothoracic ratio is an unreliable finding to suggest the presence of HF; poor inspiratory effort and fat pads around the heart may give misleading results.26
Lung ultrasound
Lung ultrasound may be used to confirm a diagnosis of acute HF, especially when NP testing is not available.6 However, it is over 40 years since pulmonary congestion on ultrasound – so-called ‘lung comets’ due to the typical ultrasound appearances – was first described,27 and it is not in routine clinical practice. Many patients without HF have lung comets on lung ultrasound,28 and it is unclear whether ultrasound is any better than a stethoscope for diagnosing pulmonary congestion.
Cardiac imaging
Cardiac imaging is essential for demonstrating abnormal cardiac structure and/or function, and thus, for making a diagnosis of HF. Imaging can also detect complications of HF, such as a LV thrombus or valve disease.
|
The current ESC 2021 guidelines and the 2023 focused update on the diagnosis and treatment of acute and chronic HF refer to the phenotypic classification to aid in determining appropriate management.6,7 |
- Transthoracic echocardiography
Echocardiography is the most commonly used imaging investigation. It is widely available, portable and relatively cheap. The combination of M-mode, 2D, spectral Doppler and colour Doppler echocardiography can provide a wealth of information on cardiac structure and function. However, almost all variables that are measured using ultrasound are prone to inter- and intra-observer error.
- Cardiac structural measurements on echocardiography
Echocardiography allows accurate measurement of:
- LV end-diastolic and end-systolic dimensions – often increased in HF (figure 6)
- LV end-diastolic and end-systolic volumes – used to calculate LVEF using Simpson’s biplane method
- left atrial (LA) size/volume – often increased in HF: LA size indexed for body surface area is a diagnostic criterion for heart failure with preserved ejection fraction (HFpEF)
- mitral valve structure – often systolic tenting or restricted posterior leaflet in ischaemic cardiomyopathy (figure 7)
- aortic, pulmonary and tricuspid valve structure
- presence and size of pleural/pericardial effusions
- size and collapsibility of the inferior vena cava (IVC) – rarely entirely normal in HF; the IVC gives a useful non-invasive indication of right atrial pressure (figure 8)
- complications of cardiac dysfunction – e.g. intracardiac thrombus (figure 9).
LVEF = End diastolic volume (ml) – End systoltic volume (ml) End diastolic volume (ml) 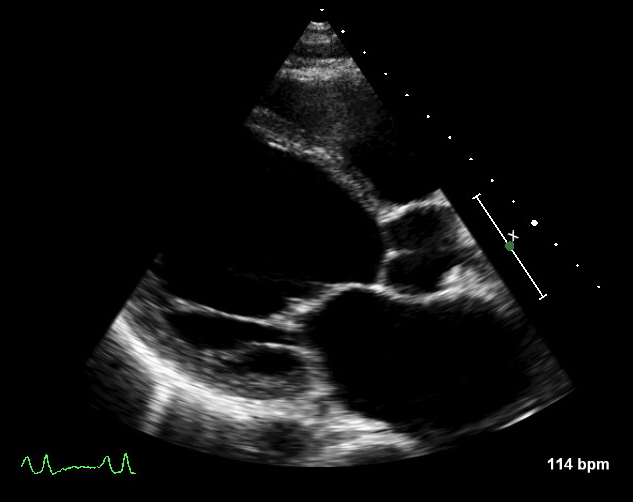
Figure 6. Echocardiogram showing dilated left ventricular end-diastolic and end-systolic dimensions in a patient with heart failure 
Figure 7. Echocardiogram showing mitral valve structure in ischaemic cardiomyopathy (Click arrow below to play, or bottom-right for full screen)

Figure 8. Echocardiogram showing inferior vena cava in a patient with heart failure (Click arrow below to play, or bottom-right for full screen)
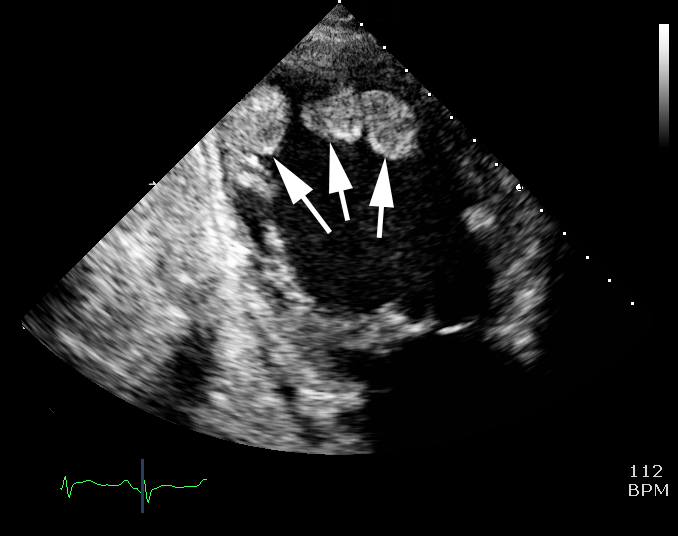
Figure 9. Echocardiogram showing intra-cardiac thrombus - Cardiac function measurements on echocardiography
Echocardiography also gives an assessment of:
- global systolic function – can be measured in various ways
- stroke volume (LV outflow tract area × LV outflow tract velocity time integral [VTI])
- cardiac output (stroke volume × heart rate)
- EF (biplane Simpson’s method)
- diastolic function (LV filling pressures)
- restrictive filling pattern on transmitral, spectral Doppler is associated with worse outcome
- longitudinal systolic and diastolic function by tissue Doppler imaging
- the ratio of the early mitral peak velocity to early diastolic mitral annular velocity is used to estimate LV filling pressure
- valvular regurgitation
- can be secondary to ventricular dilatation in HF
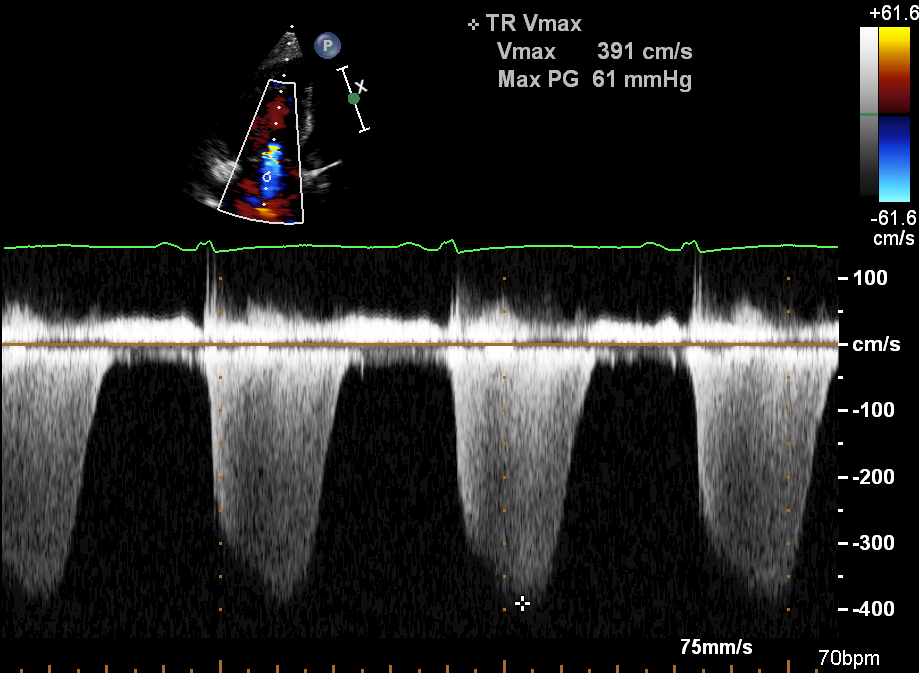
- the velocity of any tricuspid regurgitation (TR) (figure 10) allows estimation of right ventricular systolic pressure (figure 11). In combination with the IVC appearance, TR can be used to estimate pulmonary artery systolic pressure.
- global systolic function – can be measured in various ways
- Cardiac structural measurements on echocardiography
Exercise or pharmacological stress echocardiography may be used to identify the presence and extent of inducible ischaemia (evidence of myocardial ischaemia not seen at rest) and to clarify the severity of aortic stenosis.29

- Transoesophageal echocardiography
- Cardiovascular magnetic resonance scanning
Transoesophageal echocardiography (TOE) is usually not needed in routine diagnostic assessment, unless the transthoracic ultrasound window is inadequate (e.g. because of obesity or chronic lung disease, or in ventilated patients) and an alternative modality (e.g. cardiac magnetic resonance [CMR] imaging) is not available or appropriate. In special cases, such as endocarditis or complex valve disease, TOE can give more detailed information to guide diagnosis and management.
CMR scanning is becoming more widely available and can provide a range of information on different aspects of cardiac function, as well as suggest the underlying cause of HF.30 It is recommended by the ESC as the best alternative imaging modality in patients with non-diagnostic echocardiographic studies.6 For a more detailed discussion with examples, please click below.
- CMR imaging
- Anatomical thoraco-abdominal imaging:
- Cardiac anatomy, ventricular volumes, function and mass assessment:
Over approximately the past 20 years, there has been an exponential growth in the use and availability of CMR in the UK, with the most common reason for referral being the assessment of HF and cardiomyopathy.31 ESC guidelines recommend CMR in patients with inconclusive echocardiographic imaging and suspected inflammatory or infiltrative conditions.6 European registry data suggest that CMR changes patient management in approximately two-thirds of cases (for example, triggering further investigation for coronary artery disease or changing drug therapy) and is diagnostic in up to 16%.32 A typical CMR study involves the following steps:
This can be done in any plane, but typically involves a transverse stack. Figure 12 shows a patient presenting with acute pulmonary oedema in the week before the CMR study. A white blood transverse stack is shown. There is a large abdominal aortic aneurysm with an intramural thrombus. A number of renal cysts is seen. In the thorax, sternal wires are present and the LV and left atrium are dilated. The aorta follows a tortuous course.
Cine imaging can be taken in any tissue plane, typically the long axis and short axis views. Figure 13 shows the long and short axis cine images from the same patient. The LV is dilated with an indexed end-diastolic volume of 182 ml/m2 (normal: 60–95 ml/m2) and has an EF of 47%. Note the regional wall motion abnormalities and the small apical microaneurysm. The indexed LV mass is raised at 206 g/m2 (57–90 g/m2). The views of the aortic valve in figure 13A show significant aortic regurgitation (AR). A short axis cut through the valve (figures 14 and 15) shows it to be functionally bicuspid with an early aneurysm of the right sinus of Valsalva. Flow imaging found a large regurgitation fraction (51%) through the valve, confirming severe AR.



- Late gadolinium enhancement
Gadolinium is a paramagnetic transition-series element which can be used as an extracellular contrast agent in MRI. Following intravenous administration, it will accumulate in areas of the myocardium where there is expansion of the extracellular space. Expansion can occur as a result of fibrosis, infarction, oedema and infiltration. Areas of focal expansion can then be shown using CMR sequences which are particularly affected by the presence of gadolinium. These areas of abnormality appear white and are known as late gadolinium enhancement (LGE). The pattern of LGE can suggest the cause for HF and give prognostic information.
- Pathology on CMR in patients with HF
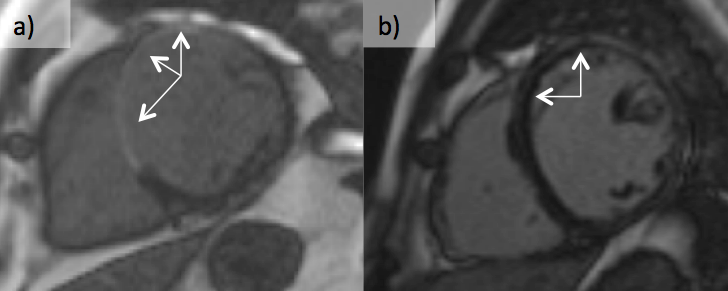
In patients with global LV dysfunction, LGE-CMR accurately differentiates ischaemic from non-ischaemic cardiomyopathy and hence, can be used as a ‘gatekeeper’ for coronary angiography.33 Areas of infarction involve the sub-endocardium, a pattern very rarely seen in non-ischaemic cardiomyopathy. In figures 14 and 15, patients with global LV impairment are shown. Figure 16a shows that the patient has transmural LGE in the septum and anterior walls. He had three-vessel disease on angiography. Figure 16b shows the patient has mid-wall and epicardial LGE. He had a family history of dilated cardiomyopathy and had a desmosomal mutation.
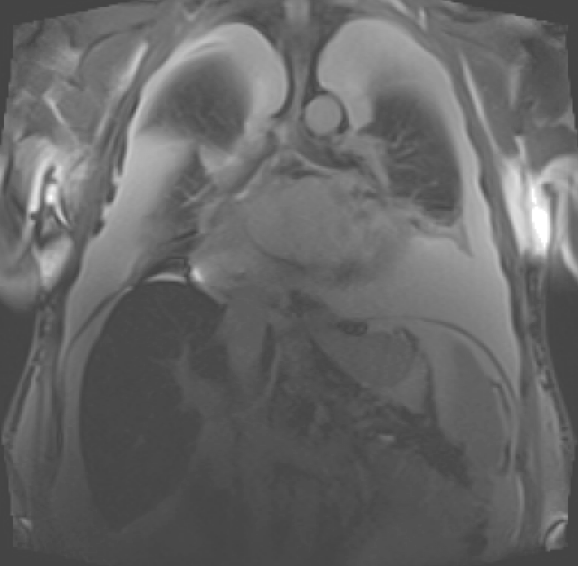
CMR can also identify other causes of a dilated LV. Figure 17a shows a coronal image from a patient presenting with severe HF, and figure 17b shows his severe biventricular dilation and dysfunction. There are bilateral large pleural effusions and the liver appears more ‘black’ than usual. These features are seen in patients with iron overload. A specific sequence, T2*, was therefore performed, which allows myocardial and liver iron quantification. There was severe iron overload in both organs. Following genotyping, a diagnosis of haemochromatosis was made and cardiac function recovered after iron chelation therapy (figure 17c).


The cine imaging of a patient presenting with a two-month history of increasing breathlessness is shown in figure 18a. There is severe biventricular failure. The LGE imaging is shown in figure 18b. There is a large amount of LGE which does not correspond with coronary artery territories, although it is transmural in places. In figure 18c, areas of lymphadenopathy are arrowed. A diagnosis of sarcoidosis was subsequently confirmed on lymph node biopsy.


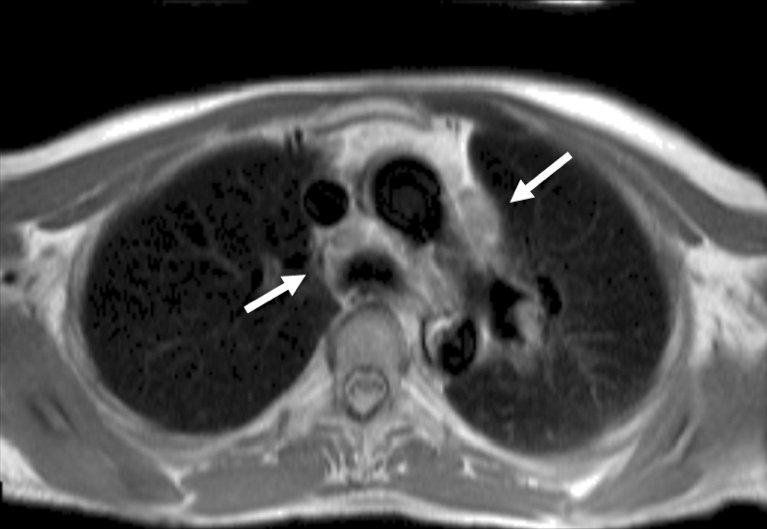
CMR also has a limited role in identifying cardiac pathology in patients with normal LV function. There are two forms of cardiac amyloidosis – AL amyloid (deposition of abnormal light chains within the myocardium) and ATTR amyloid (deposition of transthyretin – a protein responsible for transporting thyroxine and retinol) – both of which may cause HF. LGE is ubiquitous in patients with cardiac amyloidosis; the majority have transmural LGE not related to coronary artery territories.34 Other features of cardiac amyloid, such as poor longitudinal systolic function, dilated atria and LV hypertrophy are also seen on CMR. However, CMR is unable to differentiate between AL and ATTR amyloid35; further imaging is almost always needed to confirm the diagnosis (see Nuclear imaging section below).
Other imaging modalities6
Other imaging techniques can be helpful, but are not mandatory for diagnosis or monitoring of HF.1,32
- Cardiac catheterisation and coronary angiography
- Nuclear imaging
- Cardiac computed tomography
- Positron emission tomography
- DPD scanning
NICE recommends against the use of routine coronary angiography in patients with HF.5 In patients with HF and angina, other diagnostic imaging modalities are preferred – CT coronary angiogram or non-invasive functional imaging such myocardial perfusion imaging.36
However, right heart catheterisation can provide useful haemodynamic information for patients being considered for advanced therapies (LV assist devices and transplant) and can help clarify a diagnosis of pulmonary arterial hypertension, which may mimic HF.22
Nuclear imaging, including ECG-gated myocardial perfusion imaging, can be used to assess heart function and damage in HF. ECG-gated single-photon emission computer tomography (SPECT) can be used to assess global LVEF, regional wall motion abnormalities and regional wall thickening. If coronary artery disease is suspected, SPECT can also assess ischaemia and myocardial viability.
Cardiac computed tomography (CT) scanning of the heart is not usually required in the routine diagnosis and management of HF. Multidetector CT scanning is useful in delineating congenital and valvular abnormalities; however, echocardiography and CMR may provide similar information without exposing the patient to ionising radiation.
Positron emission tomography (PET) (alone or with CT) may also be used to assess ischaemia and viability, but lack of availability, radiation exposure, and wide availability of cheaper alternatives limit its use.
Tracers used in bone scanning, such as 99mTc-labelled 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), are avidly taken up by transthyretin amyloid deposits in the heart. Cardiac amyloidosis caused by accumulation of immunoglobulin light chains (AL amyloid) is not common and DPD scanning is not a sensitive way to detect it; however, ATTR is increasingly recognised as a common cause of HF in those with HF and LV hypertrophy. DPD scanning is a straightforward, sensitive and specific (and relatively cheap) way to detect it (figure 19).
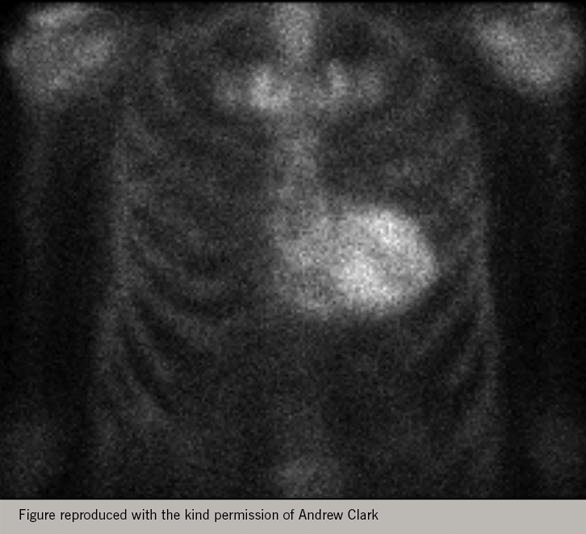
HF and myocardial viability
Cardiologists worldwide believe that improving the blood supply to areas of myocardium that are not contracting, but that are not scarred (and so classed as viable), would improve cardiac function and/or prognosis.37 Imaging tests for myocardial viability (such as dobutamine stress echocardiography or MRI) have been a common investigation for patients with HF.
Evidence
This belief was challenged by the results of the STICH (Surgical Treatment for Ischemic Heart Failure) trial, which suggested that improving blood supply to the heart by bypassing stenosed coronary arteries had no effect on symptoms or prognosis in patients with HF.38 The STICH extension study, which followed patients in the original STICH trial for up to 10 years, suggested that there was a small survival benefit from surgical revascularisation in carefully selected patients.39 Importantly, the average age of patients in STICH was only 60 years old
However, the STICH trial began recruitment approximately 20 years ago and medical therapy for HFrEF has improved enormously since. The REVIVED-BCIS II study (Revascularization for Ischemic Ventricular Dysfunction – British Cardiovascular Intervention Society II) of revascularisation using percutaneous coronary intervention (PCI) compared to medical therapy found no benefit whatsoever with revascularisation.40
Whether surgical revascularisation may lead to outcome benefit in the era of modern quadruple therapy is unknown. While viability assessment may still be performed in patients with HF, its role in decision making is diminished.
Diagnosis in the acute setting
Initial investigations should be guided by the clinical condition of the patient. Table 4 provides and overview of the pertinent tests and some examples of their findings and interpretations in the diagnostic workup of a suspected case of acute HF, based on the history, symptoms and signs. Specific investigations will be discussed in more detail later in this module.
Table 4. Diagnostic workup in suspected acute HF
| Exam | Possible findings and interpretation | ||
| Clinic room investigations | ECG | ST-segment changes, T-wave inversions and QRS changes suggest ACS/MI Rapid and irregular rate may represent AF and other tachyarrythmias Low QRS voltage, PR-segment depression, electrical alternans and sinus tachycardia – suspect cardiac tamponade41 Sinus tachycardia with RV strain, right axis deviation, S1Q3T3 sign – consider a PE |
|
| Glucose | Glucose may indicate underlying diabetes mellitus, which is common in patients with HF | ||
| Pulse oximetry | Low readings may suggest respiratory failure, large PE/pneumothorax | ||
| Imaging | Echo/FoCUS | Congestion, cardiac dysfunction, mechanical causes may be identifiable and will allow for confirmation and classification of HF | |
| CXR | Interstitial or alveolar oedema may suggest acute HF Pleural effusion may be present in acute HF or in association with other conditions such as cancer, pneumonia and tuberculosis Cardiomegaly suggests dilated cardiomyopathy or infiltrative disease Consolidation suggests pneumonia or cancer |
||
| Lung ultrasound | Kerley B lines suggest acute HF Pleural effusion could be present in acute HF, tuberculosis or cancer |
||
| Blood tests | Natriuretic peptides (pg/ml) | BNP <100 NT-proBNP <300 MR-proANP <120 |
High negative predictive value – acute HF excluded |
| BNP ≥100 NT-proBNP ≥300† MR-proANP ≥120 |
Acute HF confirmed | ||
| Serum troponin | If elevated, there is a possibility of myocardial injury i.e. ACS | ||
| FBC | Anaemia, evidence of active infection, blood dyscrasias may be detected, which can also aid to exclude precipitants and guide treatment | ||
| Creatinine and electrolytes | Renal dysfunction and electrolyte abnormalities may be detected and guide further interpretation and management | ||
| TSH | Hypo- or hyperthyroidism may be diagnosed de novo or deterioration in established disease detected | ||
| D-dimer | Elevated levels suggest a PE | ||
| Lactate | Lactic acidosis implies impaired perfusion. May be helpful when peripheral hypoperfusion is suspected | ||
| Arterial blood gas analysis | Useful to assess the type of respiratory failure and oxygenation status when respiratory failure suspected | ||
| Procalcitonin | If elevated, it can be useful to rule-in a diagnosis of pneumonia | ||
| † Rule-in values for the diagnosis of acute HF: >450 pg/mL if aged <55 years, >900 pg/mL if aged between 55 and 75 years, and >1,800 pg/mL if aged >75 years.42,43 Key: Green = investigation is recommended in all cases; Yellow = conditional recommendation; Orange = may be done but may not be necessary. ACS = acute coronary syndrome; AF = atrial fibrillation; BNP = B-type natriuretic peptide; CT = computed tomography; CT-PA = computed tomography pulmonary angiography; CXR = chest X-ray; ECG = electrocardiogram; echo = echocardiogram; FBC = full blood count; FoCUS = focused cardiac ultrasound; HF = heart failure; MR-proANP = mid-regional pro-atrial natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PE = pulmonary embolism; TSH = thyroid-stimulating hormone. |
|||
Immediate/emergency echocardiography is recommended in patients with haemodynamic instability/cardiogenic shock and in suspected life-threatening structural/functional cardiac disease (i.e. mechanical complications of acute myocardial infarction, acute valve disease, aortic dissection). Where comprehensive echocardiography is not available, point-of-care or focused cardiac ultrasound (PoCUS/FoCUS) may be used in the first instance44 with comprehensive echocardiography being performed later, though as early as possible. NICE recommends that people presenting to hospital with new suspected HF should have echocardiography within 48 hours of admission to guide early specialist treatment.13
Diagnosis in the chronic setting
The diagnosis of chronic HF requires the presence of signs and/or symptoms of HF, in conjunction with objective evidence of cardiac structural and/or functional abnormalities. The priorities for the diagnostic workup in chronic HF are illustrated in figure 20. In the chronic setting, additional focus is placed on determining the underlying aetiology and comorbidities which will also further impact management (table 5).
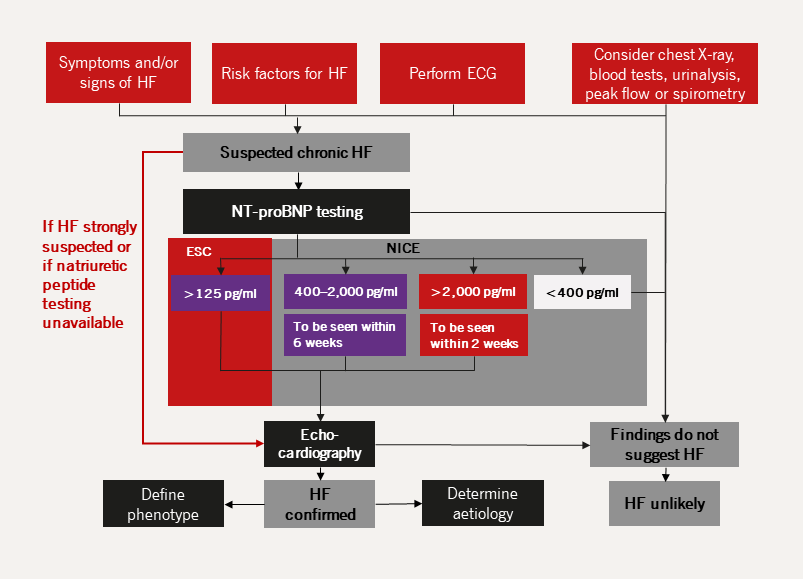
| Key: ECG = electrocardiogram; ESC = The European Society of Cardiology; HF = heart failure; NICE = The National Institute for Health and Care Excellence; NT-proBNP = N-terminal pro B-type natriuretic peptide |
Table 5. Causes and precipitants of HF, common modes of presentation and specific investigations in addition to echocardiography
| Cause | Examples of specific pathologies | Specific investigations which may be helpful |
| Coronary artery disease | MI, ACS | Invasive/CT coronary angiography Imaging stress tests (echo, nuclear, CMR) |
| Hypertension | HFpEF, malignant hypertension, acute pulmonary oedema | 24-hour ambulatory BP Plasma metanephrines Renal artery imaging Renin and aldosterone levels |
| Valve disease | Primary and secondary valve diseases e.g. aortic stenosis, functional regurgitation; congenital valve disease | Transoesophageal/stress echocardiography |
| Arrhythmias | Atrial and ventricular tachy- or bradyarrhythmias | Ambulatory ECG recording Electrophysiology study, if indicated |
| Cardiomyopathies | Dilated, restrictive, peripartum and toxin-induced cardiomyopathies; arrhythmogenic right ventricular cardiomyopathy and Takotsubo syndrome | CMR Right and left heart catheterisation Genetic testing CMR angiography Trace elements and toxin screening on hair, urine/blood tests |
| Congenital heart disease | Congenitally corrected/repaired transposition of great arteries, shunt lesions, tetralogy of Fallot and Ebstein’s anomaly | CMR |
| Infective | Viral myocarditis, Chagas disease, HIV, Lyme disease | CMR Endomyocardial biopsy Serology |
| Drug-induced | Anthracyclines, trastuzumab, VEGF inhibitors, immune checkpoint inhibitors, proteasome inhibitors, RAF+MEK inhibitors | |
| Infiltrative | Amyloid, sarcoidosis, neoplasms | Serum electrophoresis and serum free light chains Bence Jones protein Bone scintigraphy CMR CT-PET Endomyocardial biopsy Serum ACE Chest CT |
| Storage disorders | Haemochromatosis, Fabry disease, glycogen storage diseases | Iron studies Genetics CMR Endomyocardial biopsy α-galactosidase A |
| Endomyocardial disease | Radiotherapy, endomyocardial fibrosis/eosinophilia, carcinoid | CMR Endomyocardial biopsy 24-hour urine 5-HIAA |
| Pericardial disease | Calcification, infiltration | Chest CT CMR Right and left heart catheterisation |
| Metabolic | Endocrine, autoimmune or nutritional disease (thiamine, vitamin B1 and selenium deficiencies) |
TFTs Plasma metanephrines, renin and aldosterone, cortisol Specific plasma nutrients ANA, ANCA, rheumatology review |
| Neuromuscular disease | Friedreich’s ataxia, muscular dystrophy | Nerve conduction studies Electromyogram Genetics CK |
| Key: 5-HIAA = 5-hydroxyindoleacetic acid; ACE = angiotensin-converting enzyme; ANA = anti-nuclear antibody; ANCA = anti-nuclear cytoplasmic antibody; BP = blood pressure; CK = creatinine kinase; CMR = cardiac magnetic resonance; CT = computed tomography; ECG = electrocardiogram; Echo = echocardiography; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HIV = human immunodeficiency virus; LFT = liver function test; MEK = mitogen-activated protein kinase; MI = myocardial infarction; PET = positron emission tomography; RAF = rapidly accelerated fibrosarcoma; TFT = thyroid function test; VEGF = vascular endothelial growth factor | ||
Classification of HF according to LVEF (phenotypic classification)6
Heart failure with a reduced ejection fraction (HFrEF)
HFrEF is due to impaired systolic, contractile function of the LV. However, impaired systolic function does not happen in isolation – patients with HFrEF invariably also have abnormal diastolic function.
Heart failure with preserved ejection fraction (HFpEF)
Some patients have signs and symptoms of HF but have a normal EF on echocardiography (figure 21).46 Such patients are variously labelled as having normal (HFnEF) or preserved ejection fraction (HFpEF) or ‘diastolic HF’ (figure 21).
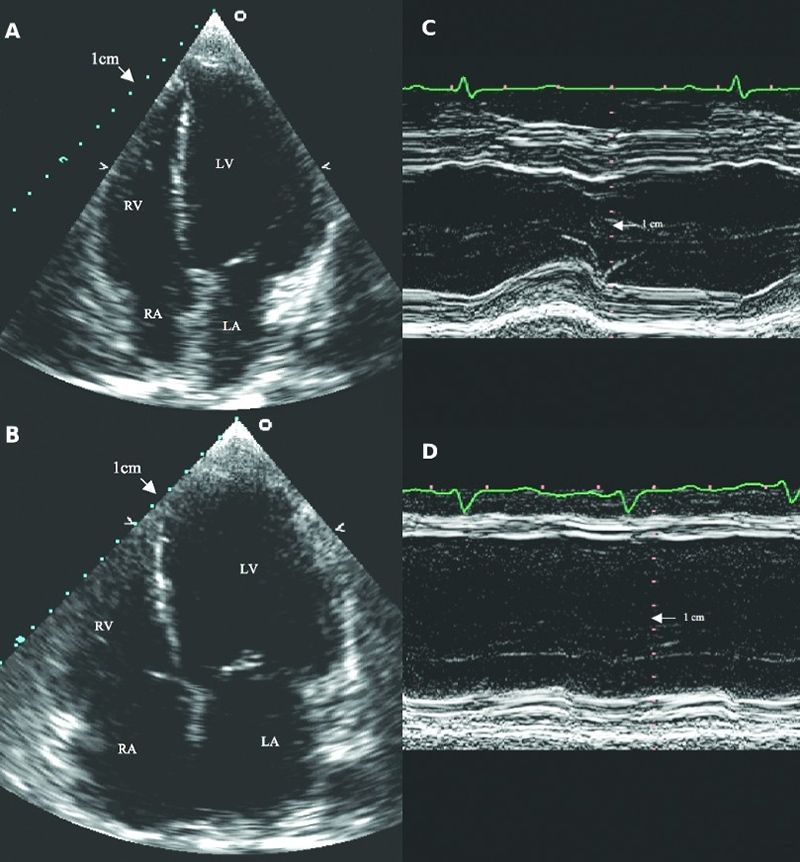
| With kind permission from Han Bin Xiao46 Key: LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle |
The EuroHeart Failure survey among women (n = 2,048; 41% of total enrolled) and men (n = 3,249; 57% of total enrolled) found that a reduced LVEF was more prevalent in men compared to women with HF (51% and 28%, respectively).47 Around half of all patients with the HF syndrome have a normal or preserved LV systolic function.47,48 When compared to patients with HFrEF, those with normal or preserved EF are usually:49,50
- older
- more likely to be female
- less likely to have coronary artery disease
- more likely to have hypertension
- more likely to have valvular heart disease
- more likely to have AF
- more likely to have diabetes or COPD
- more likely to have muscle wasting (or sarcopaenia)
- more likely to be anaemic
- more likely to have renal dysfunction
- often given different pharmacotherapy
- less likely to be admitted for HF
- less likely to respond to treatment directed at HF
- less likely to die of HF
- have a lower mortality rate.48
HFnEF remains a controversial entity; many believe that there is a significant risk of inappropriate diagnosis. The label applied can also be provocative. Many prefer the term ‘preserved’ to describe a normal EF, but this is surely a misnomer – although LVEF might be in the normal range, it might have fallen to reach that point, and cannot be known to be ‘preserved’. The ESC discussed changing HFpEF to HFnEF in its 2023 update to the 2021 ESC HF guidance but decided that any changes in its terminology would be considered in its next HF guidelines.7 Therefore, HFnEF will be referred to as HFpEF in these modules.
HFnEF
The definition of a ‘normal’ or preserved LVEF depends upon the method used to measure it and the population studied. Echocardiography is most widely used, but LVEF by echo is poorly reproducible — a patient said to have preserved LVEF by one operator may, on a different day or with a different operator, be said to have reduced EF.
Although much quoted epidemiological studies suggest that patients with HFpEF have much the same prognosis as those with HFrEF,51 every trial population in whom LVEF has been measured reports a directly proportional relationship between LVEF and survival.
An additional concern is that whilst EF may qualify as being ‘normal,’ by being above some arbitrary cut-off point, systolic function can still be abnormal when measured using other techniques, such as longitudinal strain.
Heart failure with mildly reduced ejection fraction (HFmrEF)
To complicate things further, a third HF phenotype has been proposed that lies in the ‘grey area’ between HFrEF and HFpEF: HFmrEF with LVEF 41–49%.7
The prevalence of HFmrEF is between 14% and 18% of patients with HF.52–55 Patients with HFmrEF are older and more likely to be female with greater burden of co-morbidities than patients with HFrEF, but with a similar prevalence of IHD.56 Patients with HFmrEF are thus similar to patients with HFpEF. However, their prognosis is similar to that of HFrEF and their causes of death are closer to those seen in patients with HFrEF than HFpEF.57,58
The clinical and laboratory characteristics of patients with HFmrEF are intermediate between HFpEF and HFrEF.59 It is possible that those with HFmrEF are in transition from HFpEF to HFrEF or vice versa.60–63 Although HFmrEF has become ingrained in clinical trials since it was first described in 2016, a diagnosis of HFmrEF acknowledges that the patient has HF symptoms and that the ventricular function is neither obviously normal nor obviously impaired; the utility of such a diagnosis is questionable.
Identifying the HF phenotype is essential for management
Despite much debate surrounding the value of LVEF as a measure of cardiac function, there is no doubt that it is helpful in identifying patients who are likely to respond to specific therapies. Clinical trials have unequivocally demonstrated the benefit of medical and device therapy for patients with HF, but only previously amongst those with reduced EF, whichever way that is defined.
It is important to remember that signs may not be present in the early stages of HF (especially in HFpEF) and in optimally treated patients. Therefore, in addition to symptoms, physical signs and risk factors for HF, it would be pertinent to obtain objective evidence to support the diagnosis of HF. This would include echocardiography findings (table 6)64 and NT-proBNP levels.
Although NP levels tend to be lower amongst patients with HFpEF compared to patients with HFrEF with similar symptoms of congestion,55 they cannot be used to distinguish between the two phenotypes.
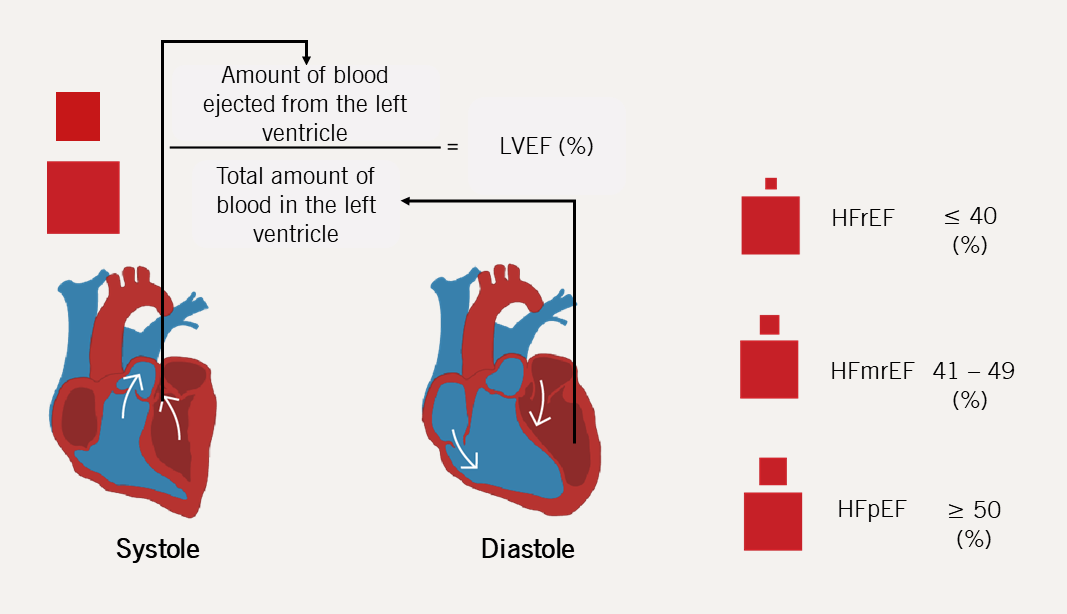
| Key: HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction |
Table 6. Objective findings to support the phenotypic classification of HF64
| Parameter | Threshold | Comments |
| LV mass index | >95 g/m2 (female) >115 g/m2 (male) |
The presence of concentric LVH is supportive, but its absence does not exclude a diagnosis of HFpEF |
| RWT | >0.42 | RWT is increased in concentric LVH. Normal RWT is 0.32–0.42, measured using the equation RWT = IVSd + PWd/LVd |
| LA volume index | >34 ml/m2 (SR) >40 ml/m2 (AR) |
In the absence of AF or valvular disease, LA enlargement reflects chronically elevated LV filling pressure |
| E/e’ ratio at rest | >9 | Sensitivity 78%, specificity 59% for HFpEF by invasive exercise testing. Cut-off of 13 has lower sensitivity (46%) but higher specificity (86%)9 |
| NT-proBNP | >125 (SR), >365 (AF) pg/ml | Up to 20% of patients with invasively proven HFpEF have NPs below diastolic thresholds, particularly in presence of obesity |
| PA systolic pressure/TR velocity at rest | >35 mmHG/>2.8 m/s | Sensitivity 54%, specificity 85% for presence of HFpEF by invasive exercise testing10 |
| ‡ For the diagnosis of HFmrEF, the presence of other evidence of structural heart disease (e.g. increased left atrial size, LV hypertrophy, or echocardiographic measures of impaired LV filling) makes the diagnosis more likely. * For the diagnosis of HFpEF, the greater the number of abnormalities present, the higher the likelihood of HFpEF Key: AF = atrial fibrillation; E/e’ = early filling velocity on transmitral Doppler/early relaxation velocity on tissue Doppler; HFpEF = heart failure with preserved ejection fraction; HFmrEF = heart failure with moderately reduced ejection fraction; IVSd = interventricular septum thickness end diastole; LA = left atrium; LV = left ventricular; LVd = left ventricular diameter end diastole; LVH = left ventricular hypertrophy; NP = natriuretic peptide; NT-proBNP = N-terminal pro B-type natriuretic peptide; PA = pulmonary artery; PWd = posterior wall thickness end diastole; RWT = relative wall thickness; TR = tricuspid regurgitation; SR = sinus rhythm |
||
Additionally, there has been a lack of clarity on how to treat patients with HFmrEF. Following the results of more recent trials, the latest ESC 2023 focused update has made some recommendations (see module 3).6,7
Summary
NP testing is key to making a diagnosis of HF but is a ‘rule-out’ test. The probability that a patient has the HF syndrome should be determined mainly by history and examination. Appropriate and timely imaging is crucial in a patient with suspected HF; it can make a diagnosis, reveal an underlying cause, guide therapy and predict outcome. However, access to diagnostic services in a timely fashion is patchy nationally and long waiting times are commonplace.
Echocardiography is the primary imaging investigation due to its widespread availability, low cost and wealth of clinical experience. CMR, however, is also a powerful imaging modality in HF which may give more information as to its aetiology.
close window and return to take test
References
- Remes J, Miettinen H, Reunanen A, Pyorala K. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315–21
- Collins SP, Lindsell CJ, Peacock WF, Eckert DC, Askew J, Storrow AB. Clinical characteristics of emergency department heart failure patients initially diagnosed as non-heart failure. BMC Emerg Med 2006;6:11 http://dx.doi.org/10.1186/1471-227X-6-11
- van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 2014;16:772–7 http://dx.doi.org/10.1002/ejhf.110
- Heilman (University of British Columbia, Canada) Photo of pitting edema during and after the application of pressure to the skin. https://en.m.wikipedia.org/wiki/File:Combinpedal.jpg
- National Institute for Health and Care Excellence. Chronic heart failure: management of chronic heart failure in adults in primary and secondary care. NICE guideline [NG106]. London: NICE, 2018. Available from: https://www.nice.org.uk/guidance/ng106 [accessed 23 October 2023]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J 2021 Oct 14]. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
- McDonagh TA, Metra M, Adamo M et al; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627–39. https://doi.org/10.1093/eurheartj/ehad195 Erratum in: Eur Heart J 2024;45:53.
- Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007;93:476–82 http://dx.doi.org/10.1136/hrt.2006.089656
- Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981;64:1227–34 https://doi.org/10.1161/01.CIR.64.6.1227
- Goode KM, Nabb S, Cleland JG, Clark AL. A comparison of patient and physician rated NYHA in a community-based heart failure clinic. J Cardiac Fail 2008;14:379–87. https:/doi.org/10.1016/j.cardfail.2008.01.014 [Epub ahead of print]
- Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202 http://dx.doi.org/10.1001/jama.289.2.194
- Roberts E, Ludman AJ, Dworzynski K et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015;350:h910. https://doi.org/10.1136/bmj.h910
- National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. Clinical guideline [CG187]. London: NICE, 2021. Available from: https://www.nice.org.uk/guidance/cg187 [accessed 23 October 2023]
- National Institute for Health and Care Excellence. Heart failure – chronic: How should I assess a person with suspected chronic heart failure? London: NICE, 2023. Available from: https://cks.nice.org.uk/topics/heart-failure-chronic/diagnosis/how-to-assess/ (accessed 01 February 2024)
- Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222
- Pellicori P, Urbinati A, Kaur K et al. Prevalence and incidence of atrial fibrillation in ambulatory patients with heart failure. Am J Cardiol 2019;124:1554–60. https://doi.org/10.1016/j.amjcard.2019.08.018 [Epub ahead of print]
- Clark AL, Goode K, Cleland JG. The prevalence and incidence of left bundle branch block in ambulant patients with chronic heart failure. Eur J Heart Fail 2008;10:696–702. https://doi.org/10.1016/j.ejheart.2008.05.001
- American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. https://doi.org/10.1164/ajrccm.166.1.at1102
- Roul G, Germain P, Bareiss P, et al. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J 1998;136:449–57
- Ingle L, Rigby AS, Carroll S, et al. Prognostic value of the 6-minute walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur Heart J 2007;28:560–8
- Cowley AJ, Fullwood L, Stainer K, Hampton JR. Exercise tolerance in patients with heart failure – how should it be measured? Eur Heart J 1991;12:50–4. https://doi.org/10.1093/oxfordjournals.eurheartj.a059824
- Callan P, Clark AL. Right heart catheterisation: indications and interpretation. Heart 2016;102:147–57. https://doi.org/10.1136/heartjnl-2015-307786 [Epub ahead of print]
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66. http://dx.doi.org/10.1016/S0140-6736(11)60101-3
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51:1073–9. http://dx.doi.org/10.1016/j.jacc.2007.10.061
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991–1001. https://doi.org/10.1016/S0140-6736(21)01754-2 [Epub ahead of print]
- Clark AL, Coats AJS. Unreliability of cardiothoracic ratio as a marker of left ventricular impairment: comparison with radionuclide ventriculography and echocardiography. Postgrad Med J 2000;76:289–91 http://dx.doi.org/10.1136/pmj.76.895.289
- Ziskin MC, Thickman DI, Goldenberg NJ, Lapayowker MS, Becker JM. The comet tail artifact. J Ultrasound Med 1982;1:1–7. https://doi.org/10.7863/jum.1982.1.1.1
- Cuthbert JJ, Pellicori P, Flockton R et al. The prevalence and clinical associations of ultrasound measures of congestion in patients at risk of developing heart failure. Eur J Heart Fail 2021;23:1831–40. https://doi.org/10.1002/ejhf.2353 [Epub ahead of print]
- Vahanian A, Beyersdorf F, Praz F et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. https://doi.org/10.1093/eurheartj/ehab395 [Erratum in: Eur Heart J 2022]
- Keenan NG, Captur G, McCann GP et al. Regional variation in cardiovascular magnetic resonance service delivery across the UK. Heart 2021;107:1974–9. https://doi.org/10.1136/heartjnl-2020-318667 [Epub ahead of print]
- Antony R, Daghem M, McCann GP, et al. Cardiovascular magnetic resonance activity in the United Kingdom: a survey on behalf of the British Society of Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson 2011;13:57. http://dx.doi.org/10.1186/1532-429X-13-57
- Bruder O, Schneider S, Nothnagel D, et al. EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol 2009;54:1457–66. http://dx.doi.org/10.1016/j.jacc.2009.07.003
- Assomull RG, Shakespeare C, Kalra PR, et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation 2011;124:1351–60. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.011346
- Martinez-Naharro A, Treibel TA, Abdel-Gadir A, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017;70:466–77. https://doi.org/10.1016/j.jacc.2017.05.053
- Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–91. https://doi.org/10.1016/j.jacc.2019.04.003
- National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Clinical guidance [CG95]. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/cg95 [accessed 02 March 2023]
- Bonow RO, Maurer G, Lee KL et al; STICH Trial Investigators. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 2011;364:1617–25. https://doi.org/10.1056/NEJMoa1100358 [Epub ahead of print]
- Carson P, Wertheimer J, Miller A, et al. The STICH Trial (Surgical Treatment for Ischemic Heart Failure). JACC Heart Fail 2013;1:400–8. http://dx.doi.org/10.1016/j.jchf.2013.04.012
- Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20 https://dx.doi.org/10.1056/NEJMoa1602001
- Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med 2022;387:1351–360. https://doi.org/10.1056/NEJMoa2206606
- Argula RG, Negi SI, Banchs J, Yusuf SW. Role of a 12-lead electrocardiogram in the diagnosis of cardiac tamponade as diagnosed by transthoracic echocardiography in patients with malignant pericardial effusion. Clin Cardiol 2015;38:139–44. https://doi.org/10.1002/clc.22370 [Epub ahead of print]
- Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330–7. https://doi.org/10.1093/eurheartj/ehi631 [Epub ahead of print]
- Januzzi JL Jr, Chen-Tournoux AA, Christenson RH, et al; ICON-RELOADED Investigators. N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. J Am Coll Cardiol 2018;71:1191–200. https://doi.org/10.1016/j.jacc.2018.01.021
- Čelutkienė J, Lainscak M, Anderson L, et al. Imaging in patients with suspected acute heart failure: timeline approach position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:181–95. https://doi.org/10.1002/ejhf.1678 [Epub ahead of print]
- Campbell P. New developments in the investigations and diagnosis of heart failure. Br J Cardio 2022;29(Suppl 2):S3–S6. https://doi.org/10.5837/bjc.2022.s06
- Xiao HB. Echocardiography in primary care. In McIntyre H (ed.). Heart failure in older patients. London: Concise Clinical Consulting, 2007.
- Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004;43:317–27. https://doi.org/10.1016/j.jacc.2003.07.046
- McDonagh TA, Gardner RS, Clark AL, Dargie H (ed.). Oxford Textbook of Heart Failure. Oxford: Oxford University Press, July 2011. http://dx.doi.org/10.1093/med/9780199577729.001.0001
- Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17:665–71. https://doi.org/10.1002/ejhf.304
- Pellicori P, Cleland JG. Heart failure with preserved ejection fraction. Clin Med (Lond) 2014;14(Suppl 6):s22–8. https://doi.org/10.7861/clinmedicine.14-6-s22
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–9. https://doi.org/10.1056/NEJMoa051530
- He KL, Burkhoff D, Leng WX, et al. Comparison of left ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40 to 55% versus <40%. Am J Cardiol 2009;103:845–51. https://doi.org/10.1016/j.amjcard.2008.11.050
- Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction. Prevalence, outcome and therapies. Circulation 2012;126:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.080770
- Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44. https://doi.org/10.1161/CIRCULATIONAHA.105.561423
- Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Col Cardiol 2007;50:768–77. https://doi.org/10.1016/j.jacc.2007.04.064 [Epub ahead of print]
- Kapoor JR, Kapoor R, Ju C, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. J Am Coll Cardiol HF 2016;4:464–72. https://doi.org/10.1016/j.jchf.2016.02.017
- Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–9. https://doi.org/10.7326/0003-4819-137-8-200210150-00006
- Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, et al. Mid-range left ventricular ejection fraction: Clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–70. https://doi.org/10.1016/j.ijcard.2017.03.032 [Epub ahead of print]
- Rickenbacher P, Kaufmann BA, Maeder MT, et al. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF). Eur J Heart Fail 2017;19:1586–96. https://doi.org/10.1002/ejhf.798
- Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63(25 Pt A):2817–27. https://doi.org/10.1016/j.jacc.2014.03.034 [Epub ahead of print]
- Clarke CL, Grunwald GK, Allen LA, et al. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes 2013;6:680–6. https://doi.org/10.1161/CIRCOUTCOMES.111.000045
- Lam CS, Teng TH. Understanding heart failure with mid-range ejection fraction. JACC Heart Fail 2016;4:473–6. https://doi.org/10.1016/j.jchf.2016.03.025
- Banerjee P. Heart failure: a story of damage, fatigue and injury? Open Heart 2017;4:e000684. https://doi.org/10.1136/openhrt-2017-000684
- Campbell P. New developments in the investigations and diagnosis of heart failure. Br J Cardiol 2022;29(suppl 2):S3-S6. https://doi.org/10.5837/bjc.2022.s06


All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.