Varicose veins and haemorrhoids both involve the venous circulatory system, but it is unclear whether they are predictors of elevated rates of other circulatory diseases. Our aim was to determine whether they are.
We analysed an epidemiological database of hospital admission and day-case statistics, constructing cohorts of people admitted for care for varicose veins or haemorrhoids, and comparing their experience of subsequent circulatory diseases with a control cohort. Compared with the control cohort, there was an elevated risk of deep vein thrombosis (DVT) in the varicose veins cohort (rate ratio 1.20; 95% confidence interval 1.08–1.33) but not in the haemorrhoids cohort (0.90; 0.78–1.03). No other circulatory diseases showed significantly elevated risks associated with varicose veins or haemorrhoids. The rate ratio for coronary heart disease in the varicose veins cohort was 0.91 (95% confidence interval 0.88–0.95) and that in the haemorrhoids cohort was 0.98 (0.94–1.03).
We conclude that neither varicose veins nor haemorrhoids showed strong association, either positive or negative, with other circulatory diseases. There was a significant, but numerically modest, elevated risk of DVT associated with varicose veins. The risk of coronary heart disease in people with varicose veins was, if anything, a bit low.
Introduction
Varicose veins (VV) are dilated, tortuous, superficial veins found typically in the lower limbs. Haemorrhoids are abnormal distensions of the arteriovenous plexus in enlarged vascular anal cushions, although they are often referred to as varicosities.1 It is unknown whether these two conditions, both classified as disorders of the venous circulatory system,2 are associated with altered risks of other circulatory disorders. If they are, this may suggest clues about shared aetiological mechanisms between VV, haemorrhoids and other circulatory diseases. It would also provide information of prognostic clinical relevance about patients with VV or haemorrhoids. We aimed to investigate whether VV and haemorrhoids are predictors of other circulatory diseases.
Methods
Population and data
We used data from the Oxford Record Linkage Study (ORLS). The ORLS includes brief statistical abstracts of records of all hospital admissions, including day-case care, in National Health Service (NHS) hospitals, and all deaths regardless of where they occurred, in defined populations within the former Oxford NHS Region, from 1963 to 1999.3
Selection of cohorts
The method will be described for the investigation of VV as a pre-existing condition and coronary heart disease (CHD) as an outcome. The same basic methods were used for studying each other circulatory disease after VV; and for studying each circulatory disease in people with haemorrhoids. A cohort of people with VV was assembled by identifying the first episode of care for VV as either a day case or inpatient at an NHS hospital. A reference cohort was constructed by identifying the first admission of individual patients in the ORLS for a range of common and generally fairly minor disorders (for details, see table footnotes). We followed the standard epidemiological practice, when hospital controls are used, of selecting a diverse range of conditions, rather than relying on a narrow range (in case the latter are themselves atypical in their risk of subsequent disease). This ‘reference’ group of conditions has been used in a number of other studies of inter-relationships between diseases.4-6 We searched the database for any subsequent NHS hospital admission or day-case care for CHD in the VV and reference cohorts. We excluded individuals for whom CHD was recorded either prior to, or at the first admission for, VV or reference condition. The diagnoses of VV, the reference conditions, and CHD were accepted as those coded on the hospital record abstract. Privacy regulations preclude access to the full records to study the criteria on which the diagnoses were based.
Statistical analysis
The methods will be described for VV followed by CHD. The methods for all other combinations of disorders were essentially the same. We calculated the rate of occurrence of CHD in the VV cohort and compared it with the rate in the reference cohort. We expressed the results as the ratio of the rate in the VV cohort compared with that in the reference cohort. The confidence interval (CI) for the rate ratio and χ2 statistics for its significance were calculated as described elsewhere.6,7 In comparing the VV cohort with the reference cohort, the rates of occurrence of CHD were standardised for age group (in five-year bands), sex, year of first admission for VV or reference condition, and district of residence, to ensure that the results of group comparisons were equivalent in these respects. We used the indirect method of standardisation, using the combined VV and reference cohort as the standard. We applied the stratum-specific rates in the combined VV and reference cohorts to the number of people in each stratum in the VV cohort, separately, and then to those in the reference cohort. This gave the standardised rate in each cohort, and thus, by comparing the rates, the means to calculate the rate ratio. In the reference cohort, we included all the people in the dataset in each stratum with the comparison conditions. We did this to maximise the numbers in each stratum in the reference cohort in order to maximise statistical power. Further detail on population and methods is described elsewhere.6
Results
There were 43,724 people in the VV cohort, 30,353 in the haemorrhoids cohort, and 464,533 in the reference cohort. In the VV cohort, 43.4% of patients were aged under 45, 43.5% aged 45–64, and 13.1% aged 65 and over. In the haemorrhoids cohort, the corresponding percentages were 42.5%, 39.7% and 17.8%, respectively (table 1). In each five-year age group, there were at least four cases in the reference cohort for each case in the VV or haemorrhoids cohort; and in many age groups there were many more.

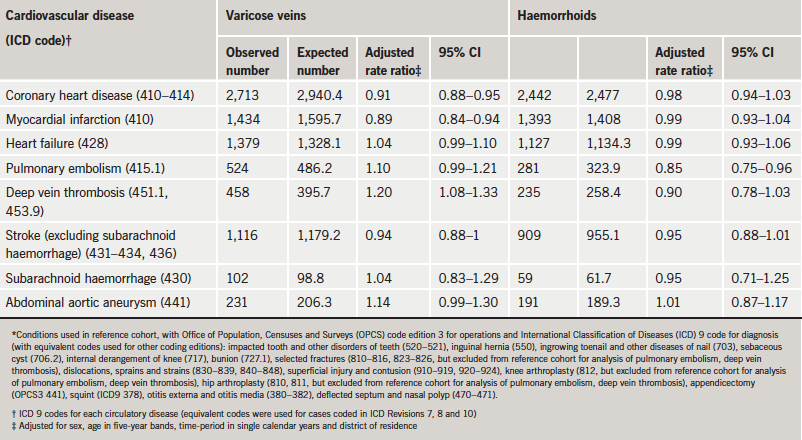
In the VV cohort, the rate ratio for CHD was a little low (0.91, compared with the reference cohort, 95% CI 0.88–0.95) (table 2). In the haemorrhoids cohort, the rate ratio for CHD was very close to one at 0.98 (95% CI 0.94–1.03). The rate ratio for deep vein thrombosis (DVT) was significantly high in the VV cohort (rate ratio 1.20; 95% CI 1.08–1.33); that for pulmonary embolism was not quite significant (rate ratio 1.10; 95% CI 0.99–1.21). The rate ratio for DVT associated with haemorrhoids was 0.90 (95% CI 0.78–1.03) and that for pulmonary embolus was borderline significantly low at 0.85 (95% CI 0.75–0.96). The rate ratio for stroke was a little low but not quite significantly so in the VV cohort (0.94; 95% CI 0.88–1.00) and the haemorrhoids cohort (0.95; 95% CI 0.88–1.01).
Comparison of males and females
In people with VV, the significantly high risk of DVT was similar for males (1.25; 95% CI 1.06–1.47) and females (1.18; 95% CI 1.02–1.36). A significantly high risk of pulmonary embolus was found for males (1.20; 95% CI 1.03–1.39) but not for females (1.03; 95% CI 0.90–1.18). The rate ratio for abdominal aortic aneurysm was significantly high in men (1.25; 95% CI 1.06–1.46) but not in women (0.88; 95% CI 0.65–1.16). There were no other significant findings, and no other noteworthy differences between men and women.
The rate ratios for most of the circulatory diseases studied in people with haemorrhoids were close to one in both males and females. The rate ratio for pulmonary embolus was significantly low in men (0.79; 95% CI 0.43–0.92), but not in women (0.93; 95% CI 0.77–1.12).
Time from admission for VV or haemorrhoids to admission for circulatory disease
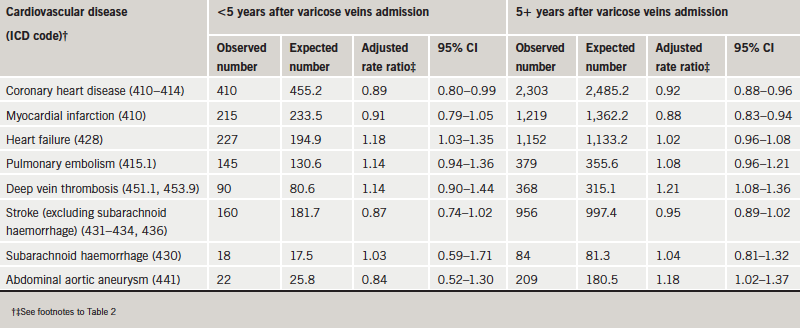
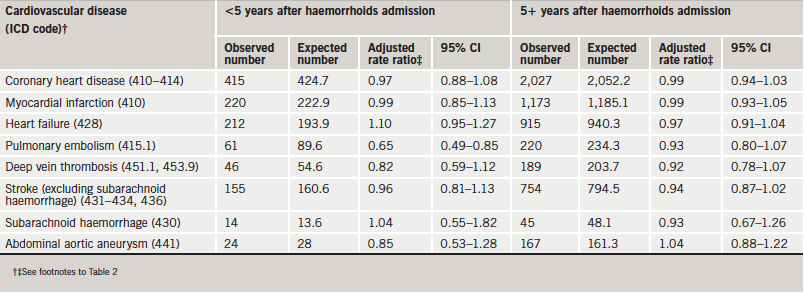
The great majority of admissions for circulatory disorders were remote in time after the admission for VV (table 3) or haemorrhoids (table 4). Following VV, the rate ratios for most circulatory disorders were very similar in the first five years after admission and at longer time intervals than that (table 3). The exception was abdominal aortic aneurysm, which showed a significantly high rate ratio more than five years after, but not in the earlier period. Following haemorrhoids, a few rate ratios that were a little low in the first five years, such as that for pulmonary embolism, became a little higher in the later period but not higher than expected from the rates in the reference cohort.
Discussion
Strengths and weaknesses
The ORLS is a large dataset containing data over a long period of time. The ‘outcome’ diagnoses, the circulatory diseases, were recorded independently of the diagnoses of VV or haemorrhoids, and only brought together by record linkage. This avoids possible biases that might arise from studies in which the observer of each condition is not ‘blind’ to the presence of other conditions. It also avoids the possibility of recall and reporting bias which might occur in retrospective interview-based studies of people who have experienced CHD, stroke or other circulatory disease. We considered the possibility of surveillance bias in which, because an individual is under care for one disease (e.g. VV), other diseases (e.g. other circulatory disorders) may be identified. The fact that the majority of the cases of circulatory diseases were remote in time – the majority occurred five years and more after VV or haemorrhoids (tables 3 and 4) – reduces the likelihood of any systematic biases related to the hospitalisation of people for VV or haemorrhoids.
Our findings were confined to people who received day-case hospital care, or who were admitted to hospital, with VV or haemorrhoids. Nonetheless, it seems reasonable to assume that those admitted are at the severe end of the clinical spectrum of VV or haemorrhoids; and that, if VV or haemorrhoids are aetiologically associated with other circulatory diseases, the associations are most likely to be evident in those with severe VV or haemorrhoids. We considered the possibility that there may be differences between males and females in indications for treatment – for example, cosmetic reasons for women and severe symptoms for men with VV – that might themselves be related to risk of other diseases. Accordingly, we analysed the data for males and females separately as well as together. However, differences between men and women were small.
We lack information about the characteristics of the patients, other than their clinical diagnoses, and therefore lack information about risk factors (such as smoking) and potential confounding for the circulatory disorders studied. Most associations were not significantly different from one, even though numbers were large and statistical power was high. Close clustering around one would not be found if the study were hampered by unmeasured confounding. Furthermore, the highest value, and the only high that was statistically significant, was the association between VV and DVT, which is also the only one where there is a reasonably strong a priori hypothesis indicating that a significant association might be found.
Varicose veins
The few previous studies of VV and CHD have produced conflicting results. The Kaiser-Permanente Epidemiologic Study of Myocardial Infarction reported that patients with VV and haemorrhoids were less likely than others to develop myocardial infarction.8 A more recent study found a reduced risk of CHD in men with VV.9 The prospective Framingham Study10 reported that, after adjusting for confounders, there were no significant associations between VV and CHD. Our study shows that in patients with pre-existing VV the risk of CHD was fractionally low.
Scott et al.9 have proposed that an inverse association between VV and CHD could be explained by increased oestrogen levels in people with VV. An alternative plausible explanation is that an inverse association may be due to differences in the smooth muscle component of the vasculature. In a histological study using VV samples from patients undergoing vein stripping, and controls from femoral-popliteal bypass operations, the former patients were demonstrated to have decreased smooth muscle in the tunica media but no difference in muscular content in other vascular wall layers.11 An in vitro study using venous samples from patients also showed decreased smooth muscle and disorganisation of the smooth muscle layers.12 In contrast to this change, it is well established that smooth muscle proliferation is a characteristic of the atherosclerotic disease process.13,14 Although it is plausible that in the above studies the controls may be unrepresentative of ‘normal’ veins, these histological changes should be considered as a potential mechanism. Future studies may be able to use control samples from young patients who have died of non-atherosclerotic related conditions.
Previous studies on the association between VV and DVT are few and, in particular, have focused on the post-operative risk of DVT in VV patients.15,16 Some case–control studies have demonstrated a positive association between VV and DVT, with similarly modest odds ratios to ours.17,18 An explanation for this association may relate to the alterations in blood flow and injuries to the vascular wall associated with VV. It is well established that one of the major complications of VV is superficial thrombophlebitis, usually with the presence of clots in the superficial venous system. It is possible, as one case series showed, that there is an increased risk of developing DVT (with or without VV) in patients with superficial vein thrombosis.19 An alternative explanation could be that some patients might develop VV subsequent to asymptomatic DVT and might thus be at elevated risk of further DVT.
Oral contraceptives have been contraindicated in patients with VV because of concerns about the possibility of an increased DVT risk, but the evidence on which this is based has been weak. A Royal College of General Practitioners study found a moderate additive effect of the presence of VV and the use of oral contraceptives on the risk of DVT, although there were only a small number of events.20 Our data provide some evidence to support a general increase in risk in people with VV. Further study is warranted to determine whether VV and other risk factors have multiplicative effects on the risk of venous thromboembolism.
Haemorrhoids
To our knowledge, this is the first large study of any association between haemorrhoids and circulatory disease. Burkitt21 in the 1970s suggested that high levels of haemorrhoids, DVT and VV in the developed world were attributable to common processes that underlie each, notably low fibre intake and lack of vigorous physical exercise. Additionally, external haemorrhoids are associated with blood stasis in the enlarged vessels, which may predispose to thromboembolic diseases. Further, in both DVT and haemorrhoids an increase in mast cells has been demonstrated,22,23 although these associations have not been adequately explored.
Conclusions
We found a statistically significant but numerically modest elevation of risk of DVT associated with VV, which is the one association studied by us that seems already to have a reasonably firm base in accepted clinical practice. Apart from this, there was no evidence that VV and haemorrhoids are associated with raised susceptibility to other circulatory diseases.
Acknowledgements
This work was supported by the English National Institute of Health Research Co-ordinating Centre for Research Capacity Development, which funds the Unit of Health Care Epidemiology to undertake research using the linked data. The Oxford Record Linkage Study dataset was built under the direction of Leicester Gill.
Conflict of interest
None declared.
Key messages
- Varicose veins and haemorrhoids both involve the venous circulatory system, but it is unclear whether they are predictors of elevated rates of other circulatory diseases
- There was a statistically significant, but numerically modest, elevated risk of deep vein thrombosis associated with varicose veins
- None of the other circulatory diseases studied showed an elevated risk in people with varicose veins or haemorrhoids
References
- Kaidar-Person O, Person B, Wexner SD. Hemorrhoidal disease: a comprehensive review. J Am Coll Surg 2007;204:102–17.
- World Health Organization. ICD chapter 2. In: International statistical classification of diseases and related health problems. 10th revision. Geneva: WHO, 1992:509–10.
- Goldacre MJ, Kurina L, Yeates D, Seagroatt V, Gill L. Use of large medical databases to study associations between diseases. QJM 2000;93:669–75.
- Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancer following hip and knee arthroplasty: record linkage study. Br J Cancer 2005;92:1298–301.
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroatt V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry 2005;187:334–8.
- Fois A, Wotton CJ, Yeates D, Turner M, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson’s disease: record linkage studies. J Neurol Neurosurg Psychiatry 2010;81:215–21.
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II – the design and analysis of cohort studies. IARC Sci Publ 1987;82:1–406.
- Klatsky AL, Friedman GD, Siegelaub AB. Medical history questions predictive of myocardial infarction: results from the Kaiser-Permanente epidemiologic study of myocardial infarction. J Chronic Dis 1976;29:683–96.
- Scott TE, Mendez MV, LaMorte WW et al. Are varicose veins a marker for susceptibility to coronary heart disease in men? Results from the Normative Aging Study. Ann Vasc Surg 2004;18:459–64.
- Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham study. Am J Prev Med 1988;4:96–101.
- Travers JP, Brookes CE, Evans J et al. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur J Vasc Endovasc Surg 1996;11:230–7.
- Lowell RC, Gloviczki P, Miller VM. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J Vasc Surg 1992;16:679–86.
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973;180:1332–9.
- Rosenfeld M, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 1990;10:680–7.
- Crandon AJ, Peel KR, Anderson JA, Thompson V, McNicol GP. Postoperative deep vein thrombosis: identifying high-risk patients. BMJ 1980;281:343–4.
- Lowe GD, Osborne DH, McArdle BM et al. Prediction and selective prophylaxis of venous thrombosis in elective gastrointestinal surgery. Lancet 1982;319:409–12.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809–15.
- Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–43.
- Chengelis DL, Bendick PJ, Glover JL, Brown OW, Ranval TJ. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
- Royal College of General Practitioners. Oral contraceptives, venous thrombosis, and varicose veins: Royal College of General Practitioners’ Oral Contraception Study. J R Coll Gen Pract 1978;28:393–9.
- Burkitt DP. Varicose veins, deep vein thrombosis, and haemorrhoids: epidemiology and suggested aetiology. BMJ 1972;2:556–61.
- Bankl HC, Grossschmidt K, Pikula B, Bankl H, Lechner K, Valent P. Mast cells are augmented in deep vein thrombosis and express a profibrinolytic phenotype. Hum Pathol 1999;30:188–94.
- Taweevisit M, Wisadeopas N, Phumsuk U, Thorner PS. Increased mast cell density in haemorrhoid venous blood vessels suggests a role in pathogenesis. Singapore Med J 2008;49:977–97.