The dipeptidyl peptidase-4 (DPP-4) inhibitors are a new class of oral drugs for the treatment of type 2 diabetes. They inhibit the breakdown of glucagon-like peptide-1 (GLP-1) and increase the incretin effect in patients with type 2 diabetes. In clinical practice they are associated with significant reductions in HbA1c, no weight gain and a low risk of hypoglycaemia. Initial cardiovascular safety studies have shown no increase in cardiovascular risk. Indeed, the suggestion of possible cardiovascular benefit seen in the safety studies is now being formally examined in large randomised-controlled trials with primary cardiovascular end points.
Introduction
 Type 2 diabetes, which is increasing in prevalence, is a major risk factor for cardiovascular morbidity and mortality. Although there are a number of pharmacological approaches to the management of type 2 diabetes, a large number of patients fail to reach glycaemic targets and a limited amount of drugs have shown benefit without glycaemic control. Therefore, there is still an unmet need in this therapeutic area.
Type 2 diabetes, which is increasing in prevalence, is a major risk factor for cardiovascular morbidity and mortality. Although there are a number of pharmacological approaches to the management of type 2 diabetes, a large number of patients fail to reach glycaemic targets and a limited amount of drugs have shown benefit without glycaemic control. Therefore, there is still an unmet need in this therapeutic area.
One recent advance in the management of type 2 diabetes has been the development and clinical use of dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists.1 The development of these two classes of drugs was based upon observations from the early twentieth century when it was noted that factors secreted from the gut participated in the regulation of pancreatic endocrine secretion. These factors were collectively termed ‘incretins’. When insulin assays became widely available in the 1960s, it became evident that the insulin secretory response to an oral glucose load was greater than when an identical amount was administered parenterally. This phenomenon was termed the incretin response. This review examines the role of DPP-4 inhibitors in clinical practice, and the role of the GLP-1 receptor agonists will be covered in the next review in this series.
Pharmacology
In humans, two incretin peptide hormones have been identified. Glucose-dependent insulin-releasing polypeptide (GIP) and GLP-1 are both secreted in response to food ingestion and potentiate the glucose-induced insulin response (figure 1). These hormones are secreted from intestinal mucosal cells, and GLP-1 can be detected in the circulation minutes after oral ingestion of nutrients. Both of these peptides are rapidly degraded by the enzyme DPP-4, which is widely expressed in a number of sites, including the endothelial cells of small gut arterioles. As a result, the majority of GLP-1 and GIP is inactivated before reaching the systemic circulation.
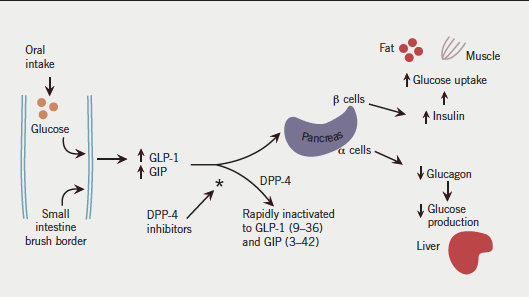
The incretin effect is diminished in type 2 diabetes.2 While secretion of GLP-1 is markedly reduced, GIP secretion remains unaffected. Administration of exogenous GLP-1 (but not GIP) leads to a significant increase in endogenous insulin secretion. GLP-1 also has important effects on reducing food intake, reduction of inappropriate glucagon production and reducing gastric emptying. Consequently, manipulating the effects of GLP-1 has been seen as having interesting potential as a therapeutic target in type 2 diabetes. At present there are two classes of drug that potentiate the incretin effect as a means of improving glycaemic control in type 2 diabetes. DPP-4 inhibitors prevent degradation of GLP-1 and increase its systemic concentration.
As the name suggests DPP-4 inhibitors inhibit the action of DPP-4, the enzyme responsible for the peripheral degradation of GLP-1. There are currently three drugs available for clinical use in this class – sitagliptin, vildagliptin and saxagliptin. In clinical trials, all three of these drugs have been shown to reduce HbA1c by 0.6–0.7% when used either as monotherapy or in combination with metformin, sulphonylureas or a combination of both. The rates of hypoglycaemia associated with these drugs are 6–10 times lower than with sulphonylureas. They have also been shown to be weight neutral. In England and Wales, the National Institute for Health and Clinical Excellence (NICE) have recommended that DPP-4 inhibitors can be used as second-line therapy in combination with either metformin or a sulphonylurea, or as third-line therapy with metformin and a sulphonylurea. Scottish Intercollegiate Guidelines Network (SIGN) guidelines have suggested that they can be used to improve blood glucose control in patients with type 2 diabetes in combination with metformin as a second-line alternative to sulphonylureas where weight gain or hypoglycaemia are of concern. SIGN also recommends third-line use in combination with metformin and sulphonylureas.
DPP-4 inhibitors are well tolerated, with hypoglycaemia only occurring when used in combination with sulphonylurea therapy. The current drugs are cleared by hepatic metabolism and renal excretion. Sitagliptin and vildagliptin are not licenced for use in renal impairment, whereas saxagliptin can be used at a reduced dose. There is a suggestion from pooled data of increased rates of infections, specifically nasopharyngitis and urinary tract infections, which suggests a role for DPP-4 activity in normal immune surveillance, but whether this is clinically significant is still to be established.
Trials of safety and efficacy
Evidence for improved glycaemic control
There are now extensive literature and multiple publications concerning the efficacy and general safety of the first three DPP-4 inhibitors: sitagliptin, vildagliptin and saxagliptin. These have been pulled together in a useful meta-analysis by Monami and colleagues, published in 2010.3 In the meta-analysis they included 32 published trials and nine unpublished trials with a duration greater than 12 weeks. They confirmed that, compared with placebo, the DPP-4 inhibitors reduced HbA1c by around 0.7%. The efficacy was similar in monotherapy and in combination with other agents. When comparisons were made with other oral drugs used in type 2 diabetes, the reductions in HbA1c were comparable with glitazones, but slightly less than with metformin or sulphonylureas. There was no weight gain and a very low risk of hypoglycaemia.
Evidence for cardiovascular safety
The DPP-4 inhibitors have not demonstrated any sustained or clinically significant reduction in other cardiovascular risk factors, such as blood pressure or lipids.4 Following the rosiglitazone controversy,5 there is now a requirement from the licensing authorities, and the Food and Drug Administration (FDA) in particular, to show that therapies for diabetes do not cause unacceptable increases in cardiovascular risk.6 The data for this can come either from a pooled analysis of pre-registration trials, or a planned dedicated cardiovascular trial. An important consideration is that there need to be sufficient cardiovascular events in the pooled analysis to confirm safety. In the past, low-risk patients with type 2 diabetes were included in phase III trials, so this has meant a change in approach to include patients with type 2 diabetes with a much greater risk of cardiovascular disease, or to include patients who have already sustained a cardiovascular event.
As a class, the DPP-4 inhibitors have not shown any suggestion of cardiovascular risk. Cardiovascular events were included in the meta-analysis by Monami and colleagues.3 The risk of cardiovascular events was non-significantly reduced to 0.76 (95% confidence interval [CI] 0.46–1.28) and the risk of all-cause mortality was non-significantly reduced to 0.78 (95% CI 0.40–1.51). There has now been individual publication of pooled safety data for the three available DPP-4 inhibitors.7-9 For sitagliptin, no differences in cardiac events were observed compared with placebo in an analysis of 5,429 patients,7 and similar results were found for vildagliptin against all comparators in data from 7,509 patients.8 For saxagliptin, data for 4,607 patients suggested a possible reduction in cardiovascular events in saxagliptin-treated patients (relative risk 0.44, 95% CI 0.24–0.82).9
To be able to confirm possible cardiovascular benefits of DPP-4 inhibitors requires large, double-blind, randomised trials with formal adjudication of cardiovascular end points. The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) is a study comparing sitagliptin with placebo in diabetic patients with a previous history of cardiovascular disease.10 It aims to recruit 14,000 subjects. The primary outcome is a composite cardiovascular end point, and it hopes to show non-inferiority with a conditional secondary superiority analysis. The Saxagliptin Assessment of Vascular Outcomes (SAVOR-TIMI 53) study is a similar study in 12,000 patients comparing saxagliptin with placebo in diabetic patients with high cardiovascular risk. The primary outcome is also a composite cardiovascular end point, and the aim is to show superiority over placebo.
Discussion
The clinical management of patients with type 2 diabetes has to balance the potential benefits of controlling hyperglycaemia on microvascular and macrovascular complications,11 with possible side effects of treatment and possible harm from over intensive control of glycaemia.12 From the perspective of the patient with diabetes, weight gain and hypoglycaemia are undesirable and unwanted side effects. Of the older therapies, sulphonylureas, insulin and glitazones are all associated with significant weight gain, whereas metformin is associated with slight weight loss. As a class, the DPP-4 inhibitors are weight neutral, causing neither weight gain nor weight loss. As the majority of patients with type 2 diabetes are overweight or obese this offers a potential advantage over older, established therapies, and DPP-4 inhibitors are being used in clinical practice as second-line therapy in addition to metformin in overweight and obese patients who fail to reach glycaemic targets with metformin monotherapy. The very low rates of hypoglycaemia are an additional advantage in vulnerable patients, such as the elderly or patients who live alone.
The preliminary cardiovascular safety data for DPP-4 inhibitors are promising, and, if the results of long-term cardiovascular studies demonstrate reductions in hard
cardiovascular end points, then this may consolidate the position of the DPP-4 inhibitors as the second-line choice for combination with metformin.
Conflict of interest
MF has served on advisory boards for MSD, Novartis, Bristol Myers Squibb and AstraZeneca Alliance, and Boehringer Ingelheim. GMck has served on advisory boards for Boehringer Ingelheim. CMcD: none declared.
Editors’ note
The next article in this series will look at the GLP-1 antagonists.
Key messages
- Dipeptidyl peptidase-4 (DPP-4) inhibitors are a new class of oral drugs for type 2 diabetes that inhibit the breakdown of glucagon-like peptide-1 (GLP-1) and increase the release of insulin in response to a meal
- In clinical practice, they do not cause weight gain and are weight
neutral, with a very low incidence of hypoglycaemia as a side effect - Results of cardiovascular safety studies have not shown any indication of cardiovascular harm, with possible suggestion of cardiovascular benefit
- The results of long-term safety studies and cardiovascular end point studies will determine the future role of these drugs
References
- Scheen AJ, Radermecker RP. Addition of incretin therapy to metformin in type 2 diabetes. Lancet 2010;375:1410–12.
- Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic individuals. J Clin Invest 1967;46:1954–62.
- Monami M, Iacomelli I, Marchionni N, Mannucci E. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2010;20:224–35.
- Verge D, Lopez X. Impact of GLP-1 and GLP-1 receptor agonists on cardiovascular risk factors in type 2 diabetes. Curr Diabetes Rev 2010;6:191–200.
- McGrane D, McKay GA, Fisher M. Drugs for diabetes: part 3 thiazolidendiones. Br J Cardiol 2011;18:24–7.
- Drucker DJ, Goldfine AB. Cardiovascular safety and diabetes drug development. Lancet 2011;377:977–9.
- Williams-Herman D, Engel SE, Round E et al. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord 2010;10:7.
- Schweizer A, Dejager S, Foley JE et al. Assessing the cardio-cerebrovascular safety of vildagliptin; meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes Obes Metab 2010;12:485–94.
- Frederich R, Alexander JH, Fiodereck FT et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med 2010;122:16–27.
- University of Oxford. Diabetes Trials Unit. Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Available from: www.dtu.ox.ac.uk/tecos [accessed 3 April 2011].
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
- The ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
