Anticoagulant therapy plays a key role in pharmacological reperfusion therapy for acute ST segment elevation myocardial infarction (STEMI). Until recently, the established role of unfractionated heparin (UFH) was unquestioned, but large trials with new agents including factor Xa inhibitors, direct thrombin inhibitors, and in particular, low molecular weight heparins (LMWHs), have shown potential advantages compared with UFH. This paper reviews the evidence base for the newer anticoagulants, with a focus on LMWH including the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment – Thrombolysis in Myocardial Infarction (ExTRACT TIMI)-25 study, which found that enoxaparin when appropriately adjusted for weight, age and renal function, provided superior net clinical benefit (balancing efficacy and safety) compared with UFH. In addition, new data from higher risk subgroups (the elderly, those with renal dysfunction or undergoing early coronary intervention) and the efficacy and safety of using concurrent clopidogrel are discussed to illustrate how these data may be integrated into contemporary practice.
Introduction
Culprit artery reperfusion with fibrinolytic therapy and/or percutaneous coronary intervention (PCI) is the established treatment for ST-elevation myocardial infarction (STEMI), with rapid access to optimised treatment providing the best outcome.1,2 Although timely primary PCI compared with fibrinolytic therapy for STEMI has demonstrated potential benefits in mortality and morbidity1 and pilot primary PCI services are currently being evaluated around the UK, fibrinolytic therapy remains the most common form of reperfusion treatment. An area of ongoing research is the optimisation of adjuvant treatment, in particular the anticoagulant agents. Historically, unfractionated heparin (UFH) has been the most commonly employed anticoagulant and is still a guideline-recommended strategy in patients treated with fibrinolytic therapy1,2 or in those undergoing primary PCI.3 However, 30-day morbidity and mortality rates with UFH remain substantial4-11 and practical limitations include the need to frequently monitor and adjust dosage; difficulty maintaining target range;10 and association with heparin-induced thrombocytopenia. Therefore, a number of alternative anticoagulant agents have been investigated in the STEMI population, including the factor Xa inhibitor fondaparinux, the direct thrombin inhibitor bivalirudin, and low molecular weight heparin (LMWH).
Factor Xa inhibitors
Fondaparinux is a synthetic pentasaccharide that selectively binds to antithrombin, potentiating by ~300 times the antithrombin mediated inhibition of factor Xa, but is devoid of direct thrombin inhibition. Potential advantages of fondaparinux over UFH include good bioavailability after subcutaneous (s/c) injection, a half-life of 15–18 hours allowing once-daily administration and no association with heparin-induced thrombocytopenia. Fondaparinux was compared with UFH in the Sixth Organisation to Assess Strategies in Ischaemic Syndromes (OASIS-6) trial,12 which enrolled 12,092 STEMI patients, grouped into two strata. Stratum-1 patients with ‘no indication for UFH’ (local investigator’s judgement, n=5,658) including some receiving streptokinase, were randomised to fondaparinux or placebo for ≤8 days. Stratum-2 patients with an ‘indication for UFH’ (n=6,434), for example those undergoing primary PCI or receiving fibrin-specific fibrinolytics, were randomised to fondaparinux/placebo for ≤8 days or UFH/placebo for 24–48 hours.
Fibrinolytic therapy was given in 45% of patients (mostly in stratum 1), primary PCI was undertaken in 28.9% (nearly all in stratum 2), and 23.7% received no reperfusion therapy. The primary end point (death/re-infarction) in stratum-1 patients was significantly reduced by fondaparinux compared with placebo (11.2% vs. 14.0%; hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.68–0.92). These stratum-1 data are of clinical importance, adding to LMWH data, showing a benefit of newer anticoagulants as adjuncts to streptokinase (a previously controversial issue with UFH).
In stratum-2 patients, there was no significant reduction in the primary end point for fondaparinux compared with UFH (8.3% vs. 8.7%; HR 0.96, 95% CI 0.81–1.13) and in patients undergoing primary PCI, there was an increase in complications with fondaparinux compared with UFH, including guide catheter thrombosis, abrupt coronary artery closure, new angiographic thrombus, or no reflow. An interim protocol modification was thus required advising additional UFH in fondaparinux patients undergoing acute PCI. Interestingly, fondaparinux compared with placebo was associated with lower rates of severe haemorrhage (28% vs. 44%; p=0.06) and major bleeds (39% vs. 57%; p=0.07) but similar bleeding compared with UFH.
In summary, factor Xa inhibitors represent an interesting new class with a convenient dosing schedule. However, while OASIS-6 showed clear benefits compared with placebo, it did not demonstrate overall benefit in 30-day efficacy or bleeding rates when compared with UFH but did raise safety concerns in patients undergoing subsequent invasive management – a finding that requires ongoing study. Current labelling restricts use to patients not thought likely to need urgent invasive management (within 120 minutes).
Direct thrombin inhibitors
Direct thrombin inhibitors inhibit thrombin (factor IIa) by specifically binding to the catalytic site and the anion-binding exosite of circulating and clot-bound thrombin. In the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes (GUSTO IIb) trial,7 2,274 STEMI patients treated with fibrinolytic therapy were randomised to the direct thrombin inhibitor hirudin or UFH. In patients receiving streptokinase, hirudin compared with UFH was associated with a reduction in 30-day death/re-infarction (8.6% vs. 14.4%; p=0.004) although no difference was seen in patients treated with tissue plasminogen activator (tPA). In the Hirudin for Improvement of Thrombolysis-4 (HIT-4) study,8 1,208 streptokinase-treated STEMI patients were randomised to recombinant hirudin or heparin. A trend to earlier ST resolution was seen, but no difference in re-infarction, mortality or bleeding rates. A similar direct thrombin inhibitor, bilvalirudin compared with UFH as an adjunct to streptokinase, showed improved early patency in the Hirolog and Early Reperfusion or Occlusion (HERO)-1 study9 and in the 17,073-patient HERO-2 study11 showed a reduction in re-infarction (odds ratio 0.70, 95% CI 0.56–0.87; p=0.001) but no significant difference in death or death/myocardial infarction (MI), and an increase in moderate bleeding. The recently reported Harmonising Outcomes with Revascularisation and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial13 in over 3,600 STEMI patients undergoing primary PCI, found bivalirudin monotherapy compared with UFH plus a glycoprotein IIb/IIIa inhibitor was associated with a reduction in net adverse clinical events (reduction in major bleeding with no excess of death/MI/ischaemic target vessel revascularisation/stroke).
While the comparability of bleeding data have been criticised given the shorter duration of bivalirudin therapy and lack of comparison with UFH monotherapy, the strategy appears promising in the primary PCI setting. However, there are no new studies currently investigating bivalirudin as an adjunct to fibrinolytic therapy in STEMI.
LMWH
LMWH are widely used in the management of unstable angina/non-STEMI, reflecting a large body of randomised data showing superiority over UFH.14,15 Large-scale randomised clinical trials and meta-analyses have now demonstrated a superior efficacy-safety balance for LMWH compared with UFH in fibrinolytic-treated STEMI patients.16,17 Based on these data, the LMWH enoxaparin was approved in 2007 for this indication by the United States Food and Drug Administration and several European countries; a decision is pending in the UK. Hence, the focus of the remainder of this review is the role of LMWH as an adjunct anticoagulant to fibrinolytic therapy.
Theoretical benefits of LMWH over UFH
The anticoagulant mechanism of heparin is based on its ability to enhance, by approximately 1000 times, the ability of antithrombin to form inhibitory complexes with thrombin, factor Xa and factor IXa.18 The finding that only a specific fraction of heparin (~30%) binds to purified antithrombin (AT or ATIII), and that this fraction possesses almost all of the anticoagulant activity of heparin,19 led to the development of LMWH. In contrast to UFH, benefits of LMWH include a higher antifactor Xa:IIa ratio, less inhibition by platelet factor 4, and an inhibition of the early rise in von Willebrand factor,20 leading to potential antiplatelet effects and a reduction in upstream thrombin generation. In addition, the high bioavailability and minimal plasma protein binding of LMWH lead to a predictable dose-response obviating the need for plasma monitoring whereas, despite regular activated partial thromboplastin time (aPTT) monitoring, UFH rarely achieves target range (<25% of cases during the initial 6–12 hours in the Thrombolysis in Myocardial Infarction [TIMI] 9B study).10
LMWH versus placebo
Four double-blind, randomised trials compared LMWH with placebo as adjunct anticoagulant in patients receiving fibrinolytic therapy for STEMI. The Fragmin in Acute Myocardial Infarction (FRAMI) trial (n=776) found dalteparin (120 IU/kg s/c twice daily) was associated with less left ventricular thrombosis/arterial thromboembolism, similar culprit artery patency but higher bleeding rates than placebo.21 Biochemical Markers in Acute Coronary Syndromes (BIOMACS-II) showed a trend to less recurrent ischaemia and better culprit artery patency at 24 hours with dalteparin than placebo.22 In the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction (CREATE)23 trial (n=15,570; 73% receiving fibrinolytic therapy) reviparin compared with placebo was associated with reduced death/re-infarction/stroke (9.6% vs. 11.0%; p=0.005), but, in those receiving fibrinolytic therapy, increased major bleeding including intracranial haemorrhage (ICH) (0.4% vs. 0.1%). In the Acute Myocardial Infarction – Streptokinase (AMI–SK) study24 (n=496) enoxaparin compared with placebo showed faster ST-segment resolution at 180 minutes, improved culprit artery patency at eight days and lower 30-day death/re-infarction/recurrent angina. Major bleeding was more common with enoxaparin (1.6% vs. 0.8%), but there was no increase in ICH (0 vs. 0.4%).
LMWH versus UFH

One study has compared dalteparin with UFH as an adjunct to fibrinolytic therapy for STEMI25 and six randomised trials provide the evidence base for enoxaparin (table 1).26-35
Phase II studies
The first data comparing LMWH with UFH were reported from a UK study in which 300 patients were randomised to enoxaparin (n=149) or UFH (n=151) for four days.26 Enoxaparin was associated with a 28% reduction in 90-day death/re-infarction/re-admission with unstable angina (26% vs. 36%; p=0.04). Multi-variate analysis identified the use of UFH rather than enoxaparin as one of only three independent predictors of the primary end point.27 There was no difference in major haemorrhage (3% vs. 4%). In the Second Trial of Heparin and Aspirin Reperfusion Therapy (HART II)28 study of 400 patients receiving recombinant tissue plasminogen activator (rt-PA), those randomised to enoxaparin compared with UFH showed a small improvement in 90-minute patency (80.1% vs. 75.1%) and less frequent re-occlusion at 5–7 days (5.9% vs. 9.8%). The Enoxaparin and TNK-tPA with or without GP IIb/IIIa Inhibitor as Reperfusion Strategy – Thrombolysis in Myocardial Infarction (ENTIRE-TIMI) 23 trial randomised nearly 500 STEMI patients receiving full-dose tenecteplase or half-dose tenecteplase plus abciximab in open-label, dose-ranging fashion to enoxaparin or UFH.29 Enoxaparin was associated with similar TIMI-3 flow rates and risk of major haemorrhage to UFH but fewer ischaemic events (30-day death/recurrent MI 4.4% vs. 15.9% in those receiving full-dose tenecteplase). The Assessment of the Safety and Efficacy of a New Thrombolytic Agent (ASSENT)-PLUS trial compared dalteparin 120 IU/kg s/c twice daily for 4–7 days with UFH 48-hour infusion as an adjunct to alteplase.25 Dalteparin reduced early coronary artery occlusion and early re-infarction, with higher TIMI flow in the infarct-related coronary artery (p=0.016). However, cessation of dalteparin therapy resulted in more re-infarction thus no between-group difference in death/re-infarction was seen by 30 days.
Phase III studies
ASSENT-3 was a phase IIIb open-label trial in which 6,095 STEMI patients receiving tenecteplase, were randomised to: (i) full-dose tenecteplase and enoxaparin (initial i.v. bolus, followed by s/c injection twice daily for ≤7 days); (ii) half-dose tenecteplase and weight-adjusted low-dose UFH and a 12-hour infusion of abciximab; or (iii) full-dose tenecteplase with weight-adjusted UFH for 48 hours.30 When given with full-dose tenecteplase, enoxaparin compared with UFH led to significant reductions in 30-day death/in-hospital re-infarction/recurrent ischaemia, with no significant increase in overall bleeding. However, the ASSENT-3 PLUS extension in 1,639 pre-hospital treated patients subsequently reported an excess of major haemorrhage and ICH in patients ≥75 years associated with enoxaparin.31 Indeed all ICH occurred in patients ≥75 years (interaction between age and bleeding risk p=0.04) suggesting dose reduction in the elderly was required. Pooled analysis of ASSENT-3 and ASSENT-3 PLUS demonstrated a benefit in the composite efficacy and safety end point for enoxaparin compared with tenecteplase,32,33 but raised safety concerns about pre-hospital use in women ≥75 years.
Meta-analysis of UFH or LMWH in STEMI
In a large meta-analysis, designed to clarify the efficacy and safety of UFH and LMWH in STEMI patients receiving fibrinolytic therapy, Eikelboom et al. found that UFH did not significantly reduce re-infarction or death compared with either no heparin or placebo, despite an increase in minor and major bleeding.16 In contrast, LMWH given for 4–8 days compared with placebo reduced death by ~10% at day 7 and day 30, and reduced re-infarction by one-quarter, albeit with an increase in minor and major bleeding. Compared with UFH, LMWH significantly reduced re-infarction by nearly 50% and non-significantly reduced death at day 7 and day 30, with an increase in minor bleeding but no significant increase in major bleeding. A recent meta-analysis of 27,131 STEMI patients confirmed the superior net clinical benefit (lower death/MI/major bleeding) with enoxaparin compared with UFH.17
Enoxaparin versus UFH

Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT)–TIMI 25, a large phase IIIb study of 20,475 STEMI patients scheduled to receive fibrinolytic therapy, investigated whether compared with UFH, weight and age (≥75 years) adjusted enoxaparin, would reduce 30-day death/MI.34,35 UFH was given as a 60 U/kg bolus, followed within 15 minutes by a 12 U/kg/hour infusion for ≥48 hours (target aPTT 1.5–2.0x control). Enoxaparin was given using a novel protocol (summarised in table 2) with a 30 mg iv bolus followed 15 minutes later by a weight-adjusted s/c dose of 1 mg/kg twice daily (first two injections maximum 100 mg). Of importance, in patients ≥75 years, the enoxaparin dosing strategy was reduced (no initial i.v. bolus, s/c dose 0.75 mg/kg, first two injections maximum 75 mg). Also, as enoxaparin is renally excreted, in patients with impaired renal function (creatinine clearance [CrCl] <30 ml/minute), the enoxaparin dose frequency was reduced to once a day.

Patients randomised to enoxaparin compared with UFH showed a 17% relative risk (RR) reduction in 30-day death/MI (9.9% vs. 12%; p<0.001 – figure 1). Major bleeding rates were lower than previous studies, although 30-day major bleeding (TIMI definition) was higher with enoxaparin (2.1 vs. 1.4%; RR 1.53, 95% CI 1.23–1.89). Notably, there was no difference in rate of ICH (0.8 vs. 0.7%; p=0.14) and the balance of efficacy and safety (net clinical benefit) significantly favoured enoxaparin over UFH. It could be argued that given the difference in treatment duration (UFH stopped after 48 hours as per American Heart Association/American College of Cardiology [AHA/ACC] guidelines, enoxaparin given a median of seven days), ExTRACT was a comparison not only of drugs but of treatment strategies. Nevertheless, at 48 hours the major secondary end point of death/MI/urgent revascularisation was already reduced with enoxaparin (6.1 vs. 5.3%; RR 0.88, 95%CI 0.79–0.98; p=0.02). While a rebound phenomenon after stopping UFH heparin is well recognised and was also seen in ExTRACT, there are no data to show continuing UFH infusion beyond 48 hours is beneficial. One-year follow-up showed the reduction of death/MI with enoxaparin remained significant (15.8 vs. 17.0%; HR 0.92, 95% CI 0.86–0.98; p<0.01).
ExTRACT and high-risk subgroups
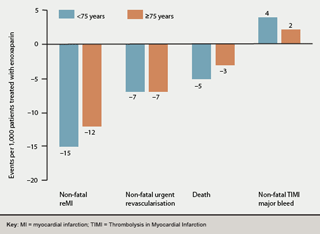
Elderly patients: Reduced enoxaparin dosing in patients ≥75 years (no i.v. bolus, 0.75 mg/kg s/c dose) and once-daily frequency in those with impaired renal function (typically more common in elderly patients) reduced the major bleeding tendency previously observed with full-dose treatment in ASSENT-3 PLUS but did not adversely affect the efficacy:safety balance, with similar absolute risk reduction and numbers needed to treat in elderly and younger patients (figure 2).36
Renal dysfunction: In ExTRACT,37 each 10 ml/minute decrease in CrCl was associated with increased death (OR 1.34; p<0.001) and 30-day death/MI (OR 1.20; p<0.001), which remained independent after adjustment for baseline variables. The treatment benefit for 30-day death/MI with enoxaparin was most marked with normal or minor renal dysfunction (CrCl >90 ml/minute: 5.1% vs. 7.3%; p<0.001, CrCl = 60–90 ml/minute: 9.6% vs. 12.1%; p<0.001) and less apparent with moderate-to-severe renal dysfunction (CrCl = 30–60 ml/minute: 19.4% vs. 19.4%; p=not significant [ns], CrCl <30 ml/minute: 33.0% vs. 37.7%; p=ns). Major bleeding rates were similar for CrCl >90 ml/minute, but more frequent in enoxaparin patients with lower CrCl.
PCI subsequent to fibrinolytic therapy: The large ExTRACT-PCI substudy38 (n=4,676) was the first to describe outcomes of patients undergoing PCI subsequent to fibrinolytic therapy in the setting of LMWH, 68% of whom received clopidogrel prior to PCI. Enoxaparin compared with UFH showed a 23% reduction in 30-day death/MI (10.7% vs. 13.8%; p=0.001), the difference emerging prior to PCI and persisting during follow-up. Interestingly, enoxaparin resulted in lower death/MI, irrespective of whether patients underwent PCI without discontinuation of study drug (14.2% vs. 18.9%; p=0.018) or had discontinued prior to PCI and then resumed anticoagulant treatment at the time of PCI (5.9% vs.14.4%; p=0.004). Fewer enoxaparin-treated patients required clinically driven urgent PCI (22.8% vs. 24.2%; p=0.027). Major (1.4% vs. 1.6%; RR 0.87) and minor bleeding rates at 30 days were similar and there was no difference in ICH rates with enoxaparin and UFH (0.2% vs. 0.4%; p=0.18). Indeed the incidence of overall stroke was lower with enoxaparin (0.3% vs. 0.9%; p=0.006).
Other subgroups: The benefit of enoxaparin was primarily seen in those achieving early complete (>70%) ST segment resolution (death/MI 2.4% vs. 6.7%; p=0.001).39 As there was no difference in rates of complete ST segment resolution this confirms the main benefit of enoxaparin is by reducing re-occlusion rather than facilitating early reperfusion. A similar mechanism probably underlies the benefit shown for adjunctive clopidogrel in the Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY) trial.40 Of relevance to UK in-hospital practice, the benefit of enoxaparin was preserved regardless of the fibrinolytic agent.41
Safety and efficacy of adjunct LMWH to fibrinolytic therapy with concurrent clopidogrel
Many UK units now routinely administer the P2Y12 adenosine-diphosphate receptor blocker clopidogrel along with aspirin and fibrinolytic therapy for STEMI given the improvement in patency and decrease in ischaemic events in the CLARITY-TIMI 28 trial42 and reduction in mortality in the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT/CCS-2) study.43 In CLARITY, adjunct anticoagulant treatment was allowed with either LMWH or UFH – a design that enabled comparison of angiographic and clinical outcomes for LMWH versus UFH in a prospective cohort of patients randomised to clopidogrel or placebo. LMWH was associated with a lower rate of death/MI/occluded infarct-related artery by discharge (13.5% vs. 22.5%, adjusted OR 0.76; p=0.027) and lower 30-day death/MI (6.9% vs. 11.5%, adjusted OR 0.68; p=0.030). Despite this augmented treatment strategy, rates of 30-day TIMI major bleeding were similar in LMWH and UFH groups (1.6% vs. 2.2%; p=0.27) and ICH (0.6% vs. 0.8%; p=0.37).44
ExTRACT-TIMI 25 enabled further assessment of combination LMWH and clopidogrel by comparing those who did (n=1,663) or did not (n=13,736) receive clopidogrel for ‘post-MI medical therapy’ at the discretion of their treating physician (excluding patients who required clopidogrel for a post-PCI indication).45 In enoxaparin patients, those also treated with clopidogrel showed 39% reduction in 30-day death/MI, while those not treated with clopidogrel showed 14% reduction (p-interaction=0.32). The absolute risk difference (ARD) in TIMI major bleeding with enoxaparin over UFH was comparable in those who were, or were not treated with clopidogrel (ARD 1.1% vs. 0.7%; p-interaction=ns) and there was no difference in ICH (ARD 0.2% vs. 0.3%; p-interaction=ns). Both substudies’ results suggest addition of clopidogrel to an anticoagulant/fibrinolytic/aspirin strategy appears safe and effective for STEMI although individual cardiac departments should evaluate the above data in the context of their current practice.
Practical implications for transition to the PCI lab
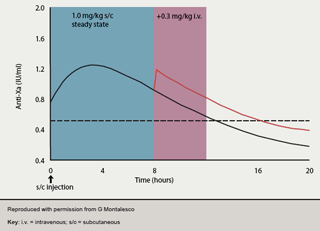
As enoxaparin becomes more widely employed for STEMI management, it is helpful to note previous Superior Yield of the New Strategy of Enoxaparin, Revascularisation and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial data,46which highlighted the importance of avoiding switching between anticoagulant agents before PCI. One approach has been to omit the previous 1–2 doses of enoxaparin and then administer conventional UFH in the cath lab. However, this leads to a period of low anticoagulant cover, pre-PCI, at a time when optimal cover would be desirable. A preferable approach may thus be to continue enoxaparin peri-procedurally as per the ExTRACT-PCI protocol. As the pharmacokinetics of enoxaparin are well understood and predictable (figure 3),47,48 no additional enoxaparin is required if the previous s/c injection has been given within eight hours. Between 8–12 hours, to maintain optimum anti-Xa levels (0.5–1.2 IU/ml), enoxaparin i.v. (0.3 mg/kg) top-up should be given. Following PCI, unless a closure device is used, the sheath should be removed ≥6 hours after the last dose of study drug. The role of LMWH in primary PCI awaits further evaluation in the Acute STEMI Treated with primary angioplasty and intravenous enoxaparin Or UFH to lower ischemic events at short and Long-term follow-up (ATOLL) trial. However, a substudy of the Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial (enoxaparin n=759; UFH n=1,693) in patients undergoing primary PCI with or without facilitation with abciximab +/- reteplase found reduced TIMI major bleeding (2.9%vs. 4.6%; p=0.043) and a trend to reduced 90-day death (3.8% vs. 5.6%; p=0.061) in those receiving enoxaparin.49
Conclusion
The role of adjunct UFH to fibrinolytic therapy in STEMI is now strongly challenged by newer anticoagulants. The evidence base is strongest for LMWH, with a strategy of enoxaparin given during the index hospitalisation associated with superior net clinical benefit to a bolus plus 48 hours infusion of UFH. Dose adjustment of enoxaparin for weight, renal function and age (≥75 years) reduces bleeding risk compared with previous studies while retaining efficacy outcomes.
Conflict of interest
IM has received honoraria from Sanofi Aventis (the manufacturers of enoxaparin) and GlaxoSmithKline (the manufacturers of fondaparinux) and has participated in clinical trials with enoxaparin, fondaparinux and bivalirudin.
Key messages
- New anticoagulants being studied in ST-elevation myocardial infarction (STEMI) include factor Xa inhibitors, direct thrombin inhibitors and low molecular weight heparin
- The Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment – Thrombolysis in Myocardial Infarction (ExTRACT-TIMI) 25 study found enoxaparin was associated with superior net clinical benefit to conventional unfractionated heparin in STEMI
- Appropriate adjustment of enoxaparin dose for age ≥75 years, weight and renal function helps minimise bleeding risks while retaining clinical efficacy
References
- Antman EM, Anbe DT, Armstrong PW et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guideline for the Management of Patients with Acute Myocardial Infarction). Circulation 2004;110:588–636.
- Van de Werf F, Ardissino D, Betriu A et al.; for the Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2003;24:28–66.
- Silber S, Albertsson P, Aviles FF et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J 2005;26:804–47.
- The International Study Group. GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Lancet 1990;336:65–71.
- ISIS-3 (Third International Study of Infarct Survival) Collaborative Group. ISIS-3: a randomized trial of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41,299 cases of suspected acute myocardial infarction. Lancet 1992;339:753–70.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82.
- The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb Investigators. A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med 1996;335:775–82.
- Neuhaus KL, Molhoek GP, Zeymer U et al. Recombinant hirudin (lepirudin) for the improvement of thrombolysis with streptokinase in patients with acute myocardial infarction: results of the HIT-4 trial. J Am Coll Cardiol1999;34:966–73.
- White HD, Aylward PE, Frey MJ et al. Randomized, double-blind comparison of hirulog versus heparin in patients receiving streptokinase and aspirin for acute myocardial infarction (HERO). Circulation 1997;96:2155–61.
- Antman EM. Hirudin in acute myocardial infarction. Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9B trial. Circulation 1996;94:911–21.
- White H. Thrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trial. Lancet 2001;358:1855–63.
- Yusuf S, Mehta SR, Chrolavicius S et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 2006;295:1519–30.
- Harmonising outcomes with revascularisation and stents in acute myocardial infarction (HORIZONS AMI). Presented at TCT2007. Available from: http://www.cardiosource.com/img/HORIZONSAMI_Slide_Set.ppt [accessed 10/12/07].
- Cohen M. The role of low-molecular-weight heparin in the management of acute coronary syndromes. J Am Coll Cardiol 2003;41:55S–61S.
- Gorog DA Kabir A, Marber M. Role of LMWH in ACS, with or without PCI and GP IIb/IIIa blockade. Br J Cardiol 2004;11:45–52.
- Eikelboom JW, Quinlan DJ, Mehta SR, Turpie AG, Menown IB, Yusuf S. Unfractionated and low-molecular-weight heparin as adjuncts to thrombolysis in aspirin-treated patients with ST-elevation acute myocardial infarction.Circulation 2005;112:3855–67.
- Murphy SA, Gibson CM, Morrow DA et al. Efficacy and safety of the low-molecular weight heparin enoxaparin compared with unfractionated heparin across the acute coronary syndrome spectrum: a meta-analysis. Eur Heart J2007;28:2077–86.
- Bjork I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem 1982:48:161–82.
- Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem 1973;248:490–505.
- Montalescot G, Philippe F, Ankri A et al.; for the French Investigators of the ESSENCE trial. Early increase of von Willebrand factor predicts adverse outcome in unstable coronary artery disease: beneficial effects of enoxaparin.Circulation 1998;98:294–9.
- Kontny F, Dale J, Abildgaard U, Pedersen TR. Randomized trial of low molecular weight heparin (Dalteparin) in prevention of left ventricular thrombus formation and arterial embolism after acute anterior myocardial infarction: the Fragmin in Acute Myocardial Infarction (FRAMI) study. J Am Coll Cardiol 1997;30:962–9.
- Frostfeldt G, Ahlberg G, Gustafsson G et al. Low molecular weight heparin (Dalteparin) as adjuvant treatment to thrombolysis in acute myocardial infarction – a pilot study: Biochemical Markers in Acute Coronary Syndromes (BIOMACS II). J Am Coll Cardiol 1999;33:627–33.
- The CREATE Trial Group Investigators. Effects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevation. JAMA2005;293:427–36.
- Simoons ML, Krzeminska-Pakula M, Alonso A et al. Improved reperfusion and clinical outcome with enoxaparin as an adjunct to streptokinase thrombolysis in acute myocardial infarction. The AMI-SK Study. Eur Heart J2002;23:1282–90.
- Wallentin L, Bergstrand L, Dellborg M et al. Low molecular weight heparin (dalteparin) compared to unfractionated heparin as an adjunct to rt-PA (alteplase) for improvement of coronary artery patency in acute myocardial infarction–the ASSENT Plus study. Eur Heart J 2003;24:897–908.
- Baird SH, McBride SJ, Trouton TG, Wilson C. LMWH versus unfractionated heparin following thrombolysis in myocardial infarction. J Am Coll Cardiol 1998;31(suppl A):191A.
- Baird SH, Menown IB, McBride SJ et al. Randomized comparison of enoxaparin with unfractionated heparin following fibrinolytic therapy for acute myocardial infarction. Eur Heart J 2002;23:627–32.
- Ross AM, Molhoek P, Lundergan C et al. A randomized comparison of low-molecular-weight heparin enoxaparin and unfractionated heparin adjunctive to t-PA thrombolysis and aspirin (HART II). Circulation 2001;104:648–52.
- Antman EM, Louwerenburg HW, Baars HF et al.; for the ENTIRE-TIMI 23 Investigators. Enoxaparin as adjunctive antithrombin therapy for ST-elevation myocardial infarction: results of the ENTIRE-Thrombolysis in Myocardial Infarction (TIMI) 23 trial. Circulation 2002;105:1642–9.
- The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet 2001;358:605–13.
- Wallentin L, Goldstein P, Armstrong PW et al. Efficacy and safety of tenecteplase in combination with the low-molecular-weight heparin enoxaparin or unfractionated heparin in the prehospital setting: the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 PLUS randomized trial in acute myocardial infarction. Circulation 2003;108:135–42.
- Armstrong PW, Chang WC, Wallentin L et al.; for the ASSENT-3 and ASSENT-3 PLUS Investigators. Efficacy and safety of unfractionated heparin versus enoxaparin: a pooled analysis of ASSENT-3 and -3 PLUS data. Can Med Assoc J 2006;174:1421–6.
- Sinnaeve PR, Huang Y, Bogaerts K et al.; on behalf of the ASSENT-3 and ASSENT-3 PLUS Investigators. Age, outcomes, and treatment effects of fibrinolytic and antithrombotic combinations: findings from Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT)-3 and ASSENT-3 PLUS. Am Heart J 2006;152:684.e1–e9.
- Antman EM, Morrow DA, McCabe CH et al.; for the EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin as antithrombin therapy in patients receiving fibrinolysis for ST-elevation myocardial infarction: design and rationale for the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-Thrombolysis in Myocardial Infarction study 25 (ExTRACT-TIMI 25). Am Heart J 2005;149:217–26.
- Antman EM, Morrow DA, McCabe CH et al.; for the ExTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006;354:1477–88.
- White HD, Braunwald E, Murphy SA et al. Enoxaparin vs. unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction in elderly and younger patients: results from ExTRACT-TIMI 25. Eur Heart J 2007;28:1066–71.
- Fox KA, Antman EM, Montalescot G et al. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol 2007;49:2249–55.
- Gibson CM, Murphy SA, Montalescot G et al. Percutaneous coronary intervention in patients receiving enoxaparin or unfractionated heparin after fibrinolytic therapy for ST-segment elevation myocardial infarction in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol 2007;49:2238–46.
- Scirica BM, Morrow DA, Giugliano RP et al. Enoxaparin Reduces Recurrent MI and Death in Patients with STEMI Undergoing Fibrinolysis who Achieve Early ST Resolution – the ExTRACT-TIMI 25 ECG Study. Circulation2006;114(suppl 2):Abstract 3487.
- Scirica BM, Sabatine MS, Morrow DA et al. The role of clopidogrel in early and sustained arterial patency after fibrinolysis for ST-segment elevation myocardial infarction: the ECG CLARITY-TIMI 28 Study. J Am Coll Cardiol2006;48:37–42.
- Giraldez RR, Nicolau JC, Corbalan R et al. Enoxaparin is superior to unfractionated heparin in patients with ST elevation myocardial infarction undergoing fibrinolysis regardless of the choice of lytic: an ExTRACT-TIMI 25 analysis.Eur Heart J 2007;28:1566–73.
- Sabatine MS, Cannon CP, Gibson CM et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179–89.
- Chen ZM, Jiang LX, Chen YP et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21.
- Sabatine MS, Morrow DA, Montalescot G et al. Angiographic and clinical outcomes in patients receiving low-molecular-weight heparin versus unfractionated heparin in ST-elevation myocardial infarction treated with fibrinolytics in the CLARITY-TIMI 28 trial. Circulation 2005;112:3846–54.
- Sabatine MS, Morrow DA, Dalby A et al. Efficacy and safety of enoxaparin versus unfractionated heparin in patients with ST-segment elevation myocardial infarction also treated with clopidogrel. J Am Coll Cardiol 2007;49:2256–63.
- Ferguson JJ, Califf RM, Antman EM et al. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004;292:45–54.
- Collet J. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation 2001;103:658–63.
- Martin JL, Fry ET, Sanderink GJ. Reliable anticoagulation with enoxaparin in patients undergoing percutaneous coronary intervention: the pharmacokinetics of enoxaparin in PCI (PEPCI) study. Catheter Cardiovasc Interv2004;61:163–70.
- The FINESSE Trial: Results of the Formal Low Molecular Weight Heparin Substudy. Presented at TCT2007. Available from: http://www.cardiosource.com/img/FINESSE_Slide_Set.ppt [accessed 10/12/07].
