We continue our series looking at pacing in patients with congenital heart disease. In the second article, we discuss the challenge of device implantation in patients with more complex congenital structural cardiac defects.
Introduction
In the previous article, we discussed those anomalies that are usually encountered by chance at, or just prior to, implantation: patent foramen ovale/atrial septal defect, Ebstein’s anomaly and ventricular septal defect, and the potential problems that they may provide to the device implanter. In this and the next article, we will discuss the challenge of device implantation in patients with more complex congenital structural cardiac defects, which the operator should be aware of prior to device implantation. In this paper we include congenitally corrected L-transposition of great arteries (L-TGA), tetralogy of Fallot and tricuspid atresia/univentricular heart, and in the final article D-transposition of the great arteries and those cases where trans-SVC pacing is practically impossible.
Congenitally corrected L-transposition of great arteries
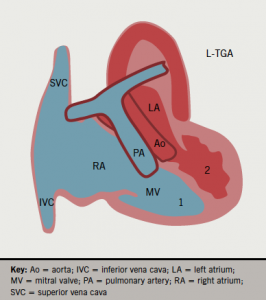
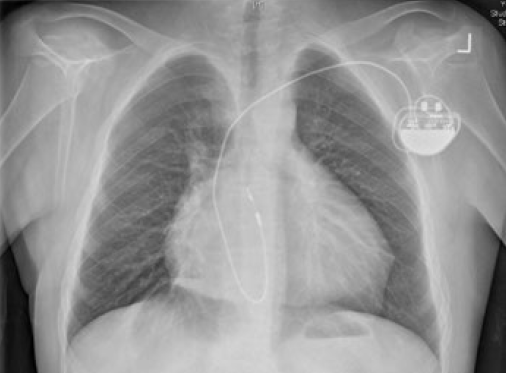
In this rare condition, a morphologic right atrium (RA) drains into a right-sided morphologic left ventricle (LV), which gives rise to the pulmonary artery (double atrioventricular and ventriculo-arterial discordance). The pulmonary venous blood enters the left atrium (LA) and then a left-sided right ventricle (RV), which functions as a systemic pumping chamber (figure 1a). In only 5% of the cases, L-TGA can be isolated (not associated with other congenital abnormality) and, therefore, asymptomatic until late adulthood. In the remaining 95% majority, it is associated with ventricular septal defect (VSD) (75%), sub/pulmonary stenosis (75%), or Ebstein-like anomaly of the tricuspid valve (75%).
Congenital atrioventricular block will occur in 25% of patients and require permanent pacing.1 Implanting physicians should understand the anatomy of this condition as ventricles lie side-by-side to each other rather than the RV being anterior to the LV in a normal heart. The septum is thus antero-posteriorly (AP) positioned rather than left to right. The atrial lead should position normally but the ventricular lead may pass inferiorly through the anatomic LV and the tip point vertically downwards (figure 1b). However, the lead tip may also point anteriorly or posteriorly, and may even point to the right on AP fluoroscopy. An intracardiac electrocardiogram (ECG) will confirm good endocardial contact. Active fixation leads are preferred because of the smaller trabeculae in the right-sided LV.
Tetralogy of Fallot
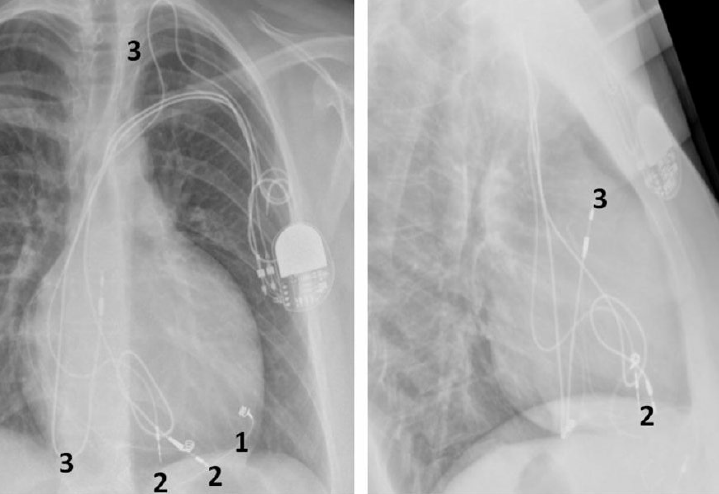
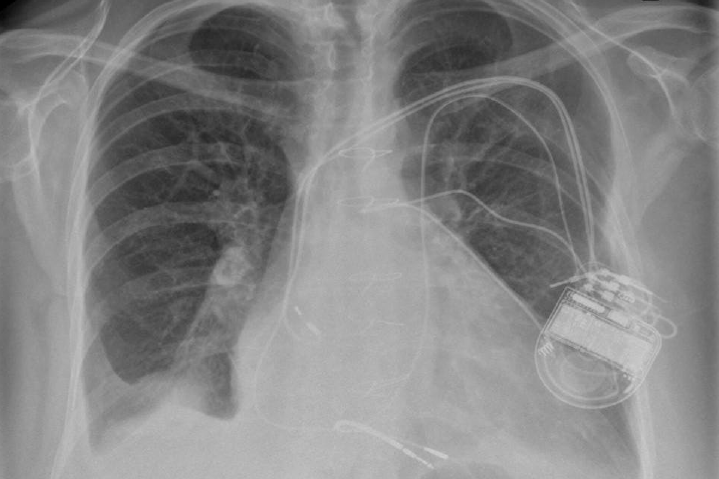
The four components of tetralogy of Fallot are: RV outflow obstruction, VSD, overriding of the aorta and RV hypertrophy. Patients with this anomaly who require permanent pacing or an implantable defibrillator (ICD) will usually have had a previous surgical procedure.2 This would usually have been a preliminary systemic-pulmonary artery anastomosis as an infant. Waterston (ascending aorta–left pulmonary artery), Cooley (ascending aorta–right pulmonary artery), Potts (descending aorta–left pulmonary artery) or Blalock-Taussig (subclavian artery–pulmonary artery) shunt are the usual temporary procedures performed prior to a more definitive intracardiac repair when the individual gets older. Surgery usually consists of patch closure of the VSD and infundibular widening using a synthetic or pericardial patch up to or across the pulmonary valve annulus. Pulmonary regurgitation and a dilated RV, prosthetic material and myocardial fibrosis make endocardial lead placement difficult and active fixation leads should be preferred (figure 2). A left superior vena cava (SVC) draining into a large coronary sinus may co-exist in 10% of patients.
Tricuspid atresia/univentricular heart
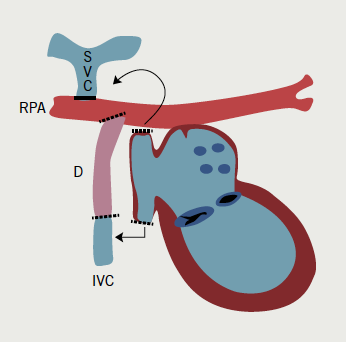
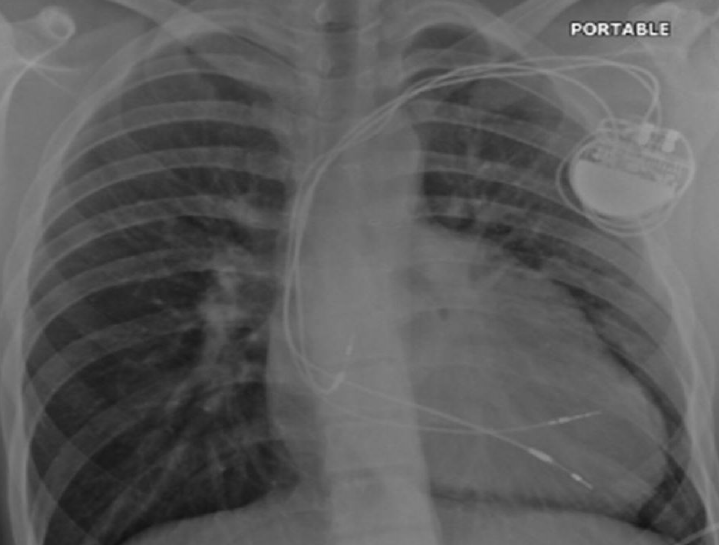
In its classic form, tricuspid atresia is best described as the absence of the right atrioventricular (AV) connection. Consequently, there must be an atrial septal defect (ASD). There is usually hypoplasia of the morphological RV, which communicates to the dominant ventricle via a VSD. Surgery to separate and redirect venous blood flow involves the Fontan repair or one of its modifications. In the classic Fontan procedure (early 1970s), the ASD is closed and a direct right atrial-pulmonary artery anastomosis is created.3,4 Frequently, the ultimately elevated atrial pressures result in severe atrial dilatation, increase in wall thickening, sinus node dysfunction, and atrial arrhythmias. Variations of the Fontan repair include a number of procedures aimed at connecting the venous circulation to the pulmonary artery by directly connecting the venae cavae to the pulmonary artery either in two steps or in one (total cavo-pulmonary anastomosis, 1990s). The same can be achieved by using an intra-atrial or, more recently, extra cardiac tunnel/conduit (figure 3a).
After the Fontan procedure (or variants) and patch closure of a VSD, atrial bradyarrhythmias and AV block are common and, therefore, pacing is indicated. Endocardial access to the RV is not possible due to the tricuspid atresia, while endocardial RA pacing is only possible if the SVC is not anastomosed to the pulmonary artery (figure 3b). Epicardial pacing is necessary in all other cases. A hybrid approach can be used in that the endocardial RA lead can be placed via the pectoral region and the epicardial ventricular lead (usually from the LV) tunnelled to the pectoral pocket for pacemaker connection (figure 3c). Alternatively, the atrial lead can be extended and tunnelled to the anterior abdominal wall and the pacemaker buried behind the rectus sheath.
Conflict of interest
None declared.
Editors’ note
Part 1 of this article was published in the last issue Br J Cardiol 2013;20:117–20. It is also available online at www.bjcardio.co.uk.
References
- Rutledge JM, Nihill MR, Fraser CD et al. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol 2002;23:137–45. http://dx.doi.org/10.1007/s00246-001-0037-8
- Hokanson JS, Moller JH. Adults with tetralogy of Fallot: long-term follow-up. Cardiol Rev 1999;7:149–55. http://dx.doi.org/10.1097/00045415-199905000-00012
- Gentles TL, Gauvreau K, Mayer JE Jr et al. Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg 1997;114:392–403. http://dx.doi.org/10.1016/S0022-5223(97)70184-3
- Gentles TL, Mayer JE Jr, Gauvreau K et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg 1997;114:376–91. http://dx.doi.org/10.1016/S0022-5223(97)70183-1
