Evidence
In the EVEREST II (Endovascular Valve Edge-to Edge Repair Study) trial, investigators compared the device to open mitral valve repair/replacement in high-risk patients with grade 3 (moderate to severe) or grade 4 (severe) mitral regurgitation.14 279 patients were randomised to percutaneous repair with the device, or open surgical repair/replacement of the valve. The primary end point for efficacy was freedom from death, from surgery for mitral-valve dysfunction, and from grade 3+ or 4+ mitral regurgitation at 12 months.
In the intention-to-treat analysis, rates of death and mitral regurgitation of grade 3+ or 4+ at 12 months were similar in both groups (6% rate of death in both groups, p=1.00, 21% in percutaneous vs. 20% in surgical group had rate of grade 3+ or 4+ mitral regurgitation), but the rate of surgery for mitral-valve dysfunction was more common in the percutaneous group (20% in percutaneous group vs. 2.2% repeat surgery in the surgery group, p<0.001). Other important findings from this trial included no statistically significant difference in rate of adverse events (excluding need for less than two units of blood transfusion which was significantly higher in the surgery group) and a significantly greater improvement of mitral regurgitation in the surgery group.
The results of this trial and other European experiences have pointed to a limited role for the device in select patients. Subgroup analysis in the EVEREST trial showed a significant benefit to patients older than 70 and importantly, in patients with functional rather than degenerative mitral regurgitation. Accordingly, this technology will likely find most use among older, high surgical risk candidates with functional mitral regurgitation.
Transcatheter mitral valve-in-valve (TMVIV) replacement
Percutaneous transcatheter mitral valve replacement (TMVR) has proven to be feasible as valve-in-valve and valve-in-ring procedure in selected high-risk surgical patients.27 TMVIV implantation is an emerging technique for reoperative mitral valve replacement in high-risk patients. Clear guidelines on the use of TMVIV implantation are yet to be determined.31
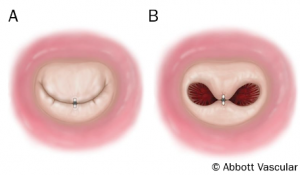
A few case series have recorded successes in the use of TMVIV technology.31-35 Cheung et al described minimal operative morbidity and mortality and favourable midterm clinical and hemodynamic outcomes in a group of 23 consecutive patients who underwent transapical TMVIV implantation for severe dysfunctional biological mitral prosthesis under transesophageal and fluoroscopic guidance (figures 11 and 12).32 Bioprosthetic dysfunction was secondary to stenosis in 6 (26.1%), regurgitation in 9 (39.1%), and combined in 8 (34.8%) patients. All patients were octogenarians with high risk for surgical redo-operation. Balloon expandable valves (Edwards Lifesciences) were implanted transapically without significant intraoperative events. Post-operatively, a significant reduction in mitral transvalvular gradient and absent mitral regurgitation (figure 13) was reported.32 Thirty-day survival was 100%. Median follow up was 753 days with 90.4% survival (NYHA class I/II). Atrial migration of transcatheter MV was reported in one patient at two months; redo-TMVIV implantation was successful. One stroke and six major bleeding events were reported in-hospital.
Figure 11. Step-by-step transapical mitral valve-in-valve procedure
Positioning (A) and deployment (B to D) of a 26-mm Edwards SAPIEN XT valve (Edwards Lifesciences) into a degenerated 27-mm Carpentier-Edwards prosthesis in mitral position
This image can be viewed as figure 1 in Cheung32
Figure 12. Panel A shows 3D transesophageal echocardiogram pre- and post-transapical mitral valve-in-valve implantation. Panel B shows systolic and diastolic 3D reconstruction of a degenerated 27-mm Carpentier-Edwards (pre) (Edwards Lifesciences) and a 26-mm Edwards SAPIEN XT (Edwards Lifesciences) valve deployed inside (post)
This image can be viewed as figure 2 in Cheung32
Figure 13. Doppler continuous transoesophageal echocardiogram showing transvalvular gradient pre- and post-transcatheter mitral valve-in-vale implantation
This image can be viewed as figure 3 in Cheung32
Similar outcomes have been demonstrated in other case series of TMVIV implantation in select high-risk groups with similar demographics and disease severity. However, some recorded a higher 30-day mortality (~7.4%).31,33-35 A case series of 19 patients even recorded successful outcomes in children as young as 10-years old.34
The available literature supports the use of transcatheter MVIV implantation in selected high-risk patients with favourable results. Although there are no available long-term data on the procedure, the early and mid-term outcomes are excellent with no evidence of structural valve deterioration in the available follow-up period.31
Key points:
- Transcatheter aortic valve implantation aims for patients with severe symptomatic aortic stenosis with predicted surgical mortality ≥15%
- Three main approaches: transfemoral, transapical, and direct aortic
- Two models: balloon-expandable and self-expanding valves
- Complications include strokes, paravalvular leaks, and vascular events
- Mitral techniques include interventions addressing the leaflets, direct annuloplasty, indirect annuloplasty (via the coronary sinus – CS), chordal implantation and left ventricular remodeling.
close window and return to take test
References
1. TAVI focus issue. Eur Heart J 2011;32. ISSN 0195-668X (Print)
2. Vahanian A, Alfieri O, Andreotti F, et al. The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J 2012;33:2451–96. http://dx.doi.org/10.1093/eurheartj/ehs109
3.Stortecky S, Buellesfeld L, Wenawesar P, Windecker S. Transcatheter aortic valve implantation: the procedure. Heart 2012;98:iv44–iv51. http://dx.doi.org/10.1136/heartjnl-2012-302401
4. Grube E, Laborde JC, Gerkens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease. The Siegburg first-in-man-study. Circulation 2006;114:1616–24. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.639450
5. Ramlawi B, Anaya-Ayala JE, Reardon MJ. Transcatheter aortic valve replacement (TAVR): access planning and strategies. Methodist Debakey Cardiovasc J 2012;8:22–5.
6.Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:Suppl:I-240–5. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.677237
7. Al Ali AM, Altwegg L, Horlick EM, et al. Prevention and management of transcatheter balloon-expandable aortic valve malposition. Catheter Cardiovasc Interv 2008:72:573–8. http://dx.doi.org/10.1002/ccd.21667
10. Piazza N, Grube E, Gerckens U, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) CoreValve Revalving System: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention 2008;4:242–9. http://dx.doi.org/10.4244/EIJV4I2A43
11. Stortecky S, Buellesfeld L, Wenawesar P, Windecker S. Transcatheter aortic valve implantation: the procedure. Heart 2012;98:iv44–iv51. http://dx.doi.org/10.1136/heartjnl-2012-302401
12. Leon MB, Smith CR, Mack M, et al. for the PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:17. http://dx.doi.org/10.1056/NEJMoa1008232
13. Kodali SK, Williams MR, Smith CR, et al. for the PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:18. http://dx.doi.org/10.1056/NEJMoa1200384
14. Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–406. http://dx.doi.org/10.1056/NEJMoa1009355
15. Perlowski A, St Goar F, Glower DG, Feldman T. Percutanenous therapies for mitral regurgitation. Curr Probl Cardiol 2012;37:42–68. http://dx.doi.org/10.1016/j.cpcardiol.2011.09.001
16. Grossi EA, Saunders PC, Woo YJ, et al. Intraoperative effects of the coapsys annuloplasty system in a randomized evaluation (RESTOR-MV) of functional ischemic mitral regurgitation. Ann Thorac Surg 2005;80:1706–11. http://dx.doi.org/10.1016/j.athoracsur.2005.04.034
17. Grossi EA, Goldberg JD, LaPietra A, et al. Ischemic mitral valve reconstruction and replacement: comparison of long-term survival and complications. J Thorac Cardiovasc Surg 2001;122:1107–24. http://dx.doi.org/10.1067/mtc.2001.116945
18. Pedersen WR, Block P, Leon M, et al. iCoapsys mitral valve repair system: percutaneous implantation in an animal model. Catheter Cardiovasc Interv 2008;72:125–31. http://dx.doi.org/10.1002/ccd.21551
19. Fukamachi K. Percutaneous and off-pump treatments for functional mitral regurgitation. J Artif Organs 2008;11:12–8. http://dx.doi.org/10.1007/s10047-007-0399-7
20. Assadi R. Percutaneous Mitral Valve Repair. http://emedicine.medscape.com/article/1839696-overview
21. Chiam PTL, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. J Am Coll Cardiol: Cardiovascular Interventions 2011;4:1–13. http://dx.doi.org/10.1016/j.jcin.2010.09.023
22. Cubeddu RJ, Palacios IF. Percutaneous techniques for mitral valve disease. Cardiol Clin, 2010;28:139–53. http://dx.doi.org/10.1016/j.ccl.2009.09.006
23. Piazza N, Asgar A, Ibrahim R, Bonan R. Transcatheter mitral and pulmonary valve therapy. J Am Coll Cardiol, 2009;53:1837–51. http://dx.doi.org/10.1016/j.jacc.2008.12.067
24. Masson JB, Webb JG. Percutaneous treatment of mitral regurgitation. Circ Cardiovasc Interv, 2009;2:140–6. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.108.837781
25. Feldman T. Percutaneous mitral valve repair. J Interv Cardiol, 2007;20:488–94. http://dx.doi.org/10.1111/j.1540-8183.2007.00295.x
26. Block PC. Percutaneous transcatheter repair for mitral regurgitation. J Interv Cardiol, 2006;19:547–51. http://dx.doi.org/10.1111/j.1540-8183.2006.00209.x
27. De Backe O, Piazza N, Banai S, et al. Percutaneous transcatheter mitral valve replacement an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv. 2014;7:400–9. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.114.001607
28. Armstrong EJ. Transcatheter mitral valve repair. http://www.uptodate.com/contents/transcatheter-mitral-valve-repair
29. Greelish JP, Cohn LH, Leacche M, et al. Minimally invasive mitral valve repair suggests earlier operations for mitral valve disease. J Thorac Cardiovasc Surg, 2003;126:365–71. Discussion: 371–3.
30. Wan B, Rahnavardi M, Tian DH, et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann Cardiothorac Surg.2013;2:6.
31. Cheung A, Al-Lawati A. Transcatheter mitral valve-in-valve implantation: current experience and review of literature. Curr Opin Cardiol. 2013;28:181–6. http://dx.doi.org/10.1097/HCO.0b013e32835cee0e
32. Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013;61:1759–66. http://dx.doi.org/10.1016/j.jacc.2013.01.058
33. Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. J Am Coll Cardiol Cardiovasc Interv. 2012;5:341–9. http://dx.doi.org/10.1016/j.jcin.2011.12.008
34. Cullen MW, Cabalka AK, Alli OO, et al. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. J Am Coll Cardiol Cardiovasc Interv. 2013;6:598–605. http://dx.doi.org/10.1016/j.jcin.2013.02.010
35. Hayek SS, Babaliaros V, Thourani V, et al. Transcatheter valve-in-valve implantation for a degenerated mitral valve bioprosthesis under echocardiographic guidance. Hellenic J Cardiol 2014;55:338–41.
36. Piazza N, Kalesan B, van Mieghem N, et al. A 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. J Am Coll Cardiol Intv. 2013;6:443–51. http://dx.doi.org/10.1016/j.jcin.2013.01.136
37. Transcatheter aortic valve replacement. Evidence and indications update. An article from the e-journal of the ESC Council for Cardiology Practice, 2013;12. http://www.escardio.org/Guidelines-&-Education/Journals-and-publications/ESC-journals-family/E-journal-of-Cardiology-Practice/Volume-12/Transcatheter-aortic-valve-replacement-Evidence-and-Indications-update
38. Latib A, Maisano F, Bertoldi L, et al. Transcatheter vs surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am Heart J. 2012;164:910–7. http://dx.doi.org/10.1016/j.ahj.2012.09.005
39. D’Errigo P, Barbanti M, Ranucci M. Transcathter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis: Results from an intermediate risk propensity-matched population of the Italian OBSERVANT study. Int J Cardiol. 2013;167:1945–52. http://dx.doi.org/10.1016/j.ijcard.2012.05.028
40. Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. Euro Intervention. 2012;8:383–9. http://dx.doi.org/10.4244/EIJV8I3A58
41. Two trials to assess TAVR in moderate-risk patients. Tctmd 2013 http://www.tctmd.com/show.aspx?id=122078
42. Haussig S, Linke A. Patient selection for TAVI 2015 – TAVI in low-risk patients: fact or fiction? Euro Intervention. 2015;Suppl W:W86–91.
Suggested further reading
Ray R, Chambers J. Mitral valve disease. Int J Clin Prac 2014;68:1216–20. http://onlinelibrary.wiley.com/doi/10.1111/ijcp.12321/epdf
Chambers J. Prosthetic valves. Int J Clin Prac 2014;68:1227–30. http://onlinelibrary.wiley.com/doi/10.1111/ijcp.12309/epdf
close window and return to take test
All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.
