The genetics of PAH
The major genetic predisposing factor is heterozygous loss of function mutations in bone morphogenetic protein receptor type 2 (BMPR2), which has been recognised in more than 75% of heritable PAH (HPAH) and up to 25% of idiopathic PAH (IPAH) cases. Some 300 BMPR2 mutations have been identified to date.18 BMPR2 encodes a type 2 receptor for bone morphogenetic proteins involved in the control of vascular cell proliferation. Mutations in activin-like kinase (ALK1), endoglin (ENG), BMPR1B and SMAD9 have been identified in patients with PAH with a personal or family history of hereditary haemorrhagic telangiectasia. Rare heterozygous mutations in genes coding for proteins such as caveolin 1 and the potassium channel subfamily K member 3 have also been identified.
The presence of a gene mutation does not necessarily mean that the individual will develop PAH during their life as the gene is thought to have only a 20% penetrance. As a consequence, PAH may jump generations. It is believed that a “second hit” in the form of an environmental trigger may be required to precipitate PAH in patients harbouring a mutation. Whilst it has been shown that there is an earlier age of diagnosis in successive generations of families with mutations, it is unclear as to whether this is due to true genetic anticipation or as a consequence of increased awareness of the disease in affected families and hence earlier presentation to medical professionals.
Furthermore, recessive transmission has been suggested in heritable PVOD/PCH. Bi-allelic mutations in eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) were present in all familial PVOD/PCH and in 25% cases of confirmed sporadic PVOD/PCH
Drug- and toxin-induced PAH
A number of drugs and toxins have been identified that have either a definite, likely or possible association in predisposing or facilitating disease development. These are summarised in table 5.
Table 5. Updated risk level of drugs and toxins known to induce pulmonary arterial hypertension
This can be viewed as table 7 in 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension
Screening for pulmonary hypertension
Increasing evidence shows that early treatment in PAH improves long term outcomes. Asymptomatic individuals who belong to groups in which PH or PAH is highly prevalent should be screened. As discussed in this module, there are several medical conditions and genetic mutations which are recognised as risk factors. This includes patients with systemic sclerosis, sickle cell disease, congenital heart disease with a shunt, HIV infection, recent pulmonary embolism, BMPR2 mutation carriers or relatives of patients with heritable PAH and patients with portal hypertension referred for liver transplantation.
A screening test should ideally be non-invasive, reproducible, cost effective and associated with a high negative predictive value for the condition. Echocardiography remains the best way to estimate elevated pulmonary pressures. Clinical judgement about when to undertake cardiac catheterisation should be used and current guidelines followed.
The recommendations for diagnostic management according to echocardiographic probability of PH in asymptomatic patients with or without risk factors for PAH or CTEPH is summarised in tables 6 and 7.
Table 6. Diagnostic management suggested according to echocardiographic probability of pulmonary hypertension in asymptomatic patients with or without risk factors for pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension
This can be viewed as web table IX in 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension – web addenda
Table 7. Recommendations for pulmonary arterial hypertension screening
This can be viewed as web table IX in 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension – web addenda
BMPR2 mutations carriers/heritable PAH
The lifetime risk of developing PAH in BMPR2 mutation carries is approximately 20%, with women being at higher risk than men. Currently, aymptomatic subjects who are mutation carriers and subjects who are first degree relatives of patients with hereditary PAH in whom no causal mutation has been identified, should be offered yearly screening echo. Ongoing longitudinal studies (DELPHI) will hopefully clarify this in the future.
SSc patients and connective tissue disease
Studies have shown that asymptomatic PAH can be missed by Doppler echocardiography in patients with SSc and a composite measure has been proposed in the recent DETECT study. However, there is no long term follow up data on the outcomes of the those patients diagnosed with asymptomatic PAH. Hence, currently, annual screening with echocardiography, diffusing capacity for carbon monoxide (DLCO) and BT-proBNP is suggested in patients with SSc or mixed connective tissue disease with U1 RNP antibodies. In patients with other connective tissue diseases such as SLE and rheumatoid arthritis, echocardiography is recommended only when symptoms develop.
Congenital heart disease
Patients who have congenital heart disease with systemic-to-pulmonary shunts are at risk of developing PAH, as a consequence of exposure of the pulmonary vessels to increased blood flow and increased pressure. At the time of diagnosis, patients should be have an echocardiogram and right heart catheterisation as considered appropriate. Please note that catheterisation is not always essential in congenital heart disease. In addition, surgical repair may be considered for patients with a significant shunt.
HIV
PAH is seen in roughly 0.5% of patients with HIV, and may be an indirect effect of the virus on inflammation and growth factors. Routine screening of asymptomatic patients is not recommended but if patients develop shortness of breath then investigation should include echocardiography. Screening is not routine in patients with HIV infection or chronic haemolytic anaemia.
Liver transplantation
The risk of developing PAH increases with duration of portal hypertension, and may be as high as 4% in patients with advanced liver disease awaiting transplantation. If transplantation is under consideration then echocardiography should be performed, and cardiac catheterisation if evidence of PAH is demonstrated (PAH is predictive of poor outcome after transplantation). Screening is recommended as part of the workup for liver transplantation.
Anorexigen associated
The development of PAH has been linked to exposure to anorexigens and structurally related compounds such as fenfluramine. Echocardiography is required only if the patient is symptomatic.
Haemoglobinopathies
Pulmonary hypertension is increasingly recognised in association with haemoglobinopathies such as sickle cell disease and thalassaemia. These patients may be offered annual echocardiography, although this is a matter of debate at the present time.
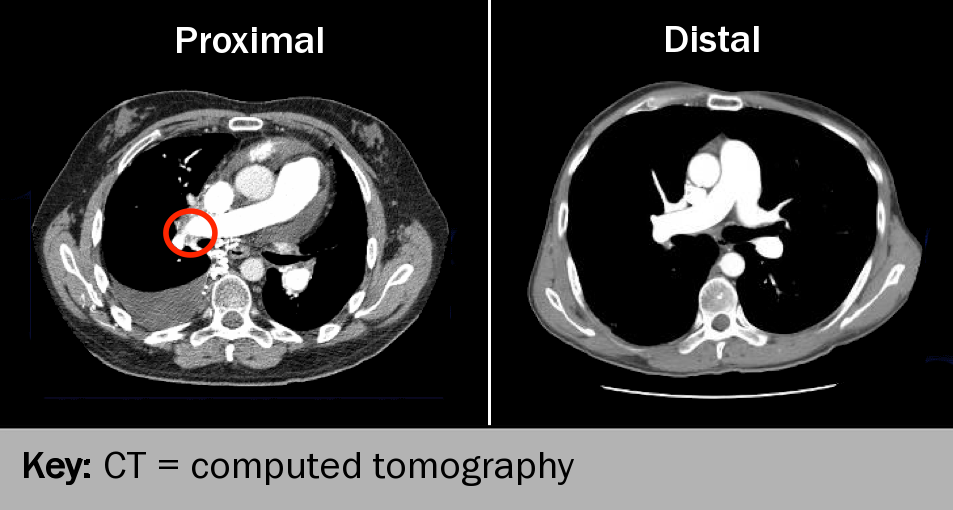
Pulmonary embolism
Some 2–4% of patients who develop an acute pulmonary embolism (PE) go on to develop chronic thromboembolic pulmonary hypertension (CTEPH) (see figure 6). Those most at risk have certain factors specific to PE (recurrent or idiopathic PE, large perfusion defects at diagnosis, young or old age at diagnosis, and echocardiographic evidence of PH at diagnosis or six months later), concurrent chronic medical conditions, associated thrombotic factors and genetic factors.24 Echocardiography may be offered to patients within six months after acute PE but the value of this remains to be proven.
Learning points
- The best approach to screening in asyptomatic populations at risk of developing PAH is unknown
- For now, for those who are at highest risk such as subjects who are mutation carriers or those with SSc, annual echocardiography is recommended
close window and return to take test
References
1 Galiè N, Humbert M, Vachiery JL et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2015;37:67–119. http://dx.doi.org/10.1093/eurheartj/ehv317
2 Gibbs, JSR, et al.. Consensus statement on the management of pulmonary hypertension in clinical practice in the UK and Ireland. Thorax 2008;63:ii1-35. http://dx.doi.org/10.1136/thx.2007.090480
3 Simonneau G, Robbins IM, Berghetti M et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54: S43–S54. http://dx.doi.org/10.1016/j.jacc.2009.04.012
4 Tuder RM, Abman SH, Braun T et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 2009;54:S3–S9. http://dx.doi.org/10.1016/j.jacc.2009.04.009
5 McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. J Am Coll Cardiol 2009;53:1573–619. http://dx.doi.org/10.1016/j.jacc.2009.01.004
6 Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10–S19. http://dx.doi.org/10.1016/j.jacc.2009.04.006
7 Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327: 70–5. http://dx.doi.org/10.1056/NEJM199207093270202
8 Rubens C, Ewert R, Halank M et al. Big endothelin-1 and endothelin-1 levels are correlated with the severity of primary pulmonary hypertension. Chest 2001;120:15662–9. http://dx.doi.org/10.1378/chest.120.5.1562
9 Bonnet S, Michelakis ED, Porter CJ, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–41. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.609008
10 Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–30. http://dx.doi.org/10.1164/rccm.200510-1668OC
11 Badesch DB, Champion HC, Gomez Sanchez M, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. http://dx.doi.org/10.1016/j.jacc.2009.04.011
12 Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis-associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 2003;62;1088–93. http://dx.doi.org/10.1136/ard.62.11.1088
13 Tapson VF, Humbert M. Incidence and prevalence of CTEPH. Proc Am Thorac Soc 2006;3:564–7.
14 National audit of pulmonary hypertension 2011. NHS Information Centre 2011. Available from https://catalogue.ic.nhs.uk/publications/clinical/heart/nati-pulm-hype-audi-2011/nati-pulm-hype-audi-2011-rep.pdf
15 World symposium on primary pulmonary hypertension 1998, held in Evian, France in 1998, and co-sponsored by the World Health Organization. Available from http://web.archive.org/web/20020408173726/http://www.who.int/ncd/cvd/pph.html
16 Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–63. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.911818
17 Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med 1987;107:216–23.
18 Machado RD, Eickelberg O, Elliott G, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54 (Suppl S):S32–S42. http://dx.doi.org/10.1016/j.jacc.2009.04.015
19 Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–44.
20 Black CM. Foreword, Consensus statement on the management of pulmonary hypertension in clinical practice in the UK and Ireland. Thorax 2008;63(Suppl II): ii1–ii41. http://dx.doi.org/10.1136/thx.2007.090480
21 Fishman AP. Clinical classification of pulmonary hypertension. Clin Chest Med 2001;22:385–91.
22 Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S.
23 Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354:579–87. http://dx.doi.org/10.1056/NEJMoa052744
24 Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2011;364:351–60. http://dx.doi.org/10.1056/NEJMra0910203
25 National Audit of Pulmonary Hypertension 2014, Health and Social Care Information Centre 2015. Available from http://www.hscic.gov.uk/catalogue/PUB17264/nati-pulm-hype-audi-2014-rep.pdf
26. Coghlan JG, Denton CP, Grünig E, et al., on behalf of the DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. http://dx.doi.org/10.1136/annrheumdis-2013-203301
27. Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005;52:3792–800. http://dx.doi.org/10.1002/art.21433
28. Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011;63:3522–30. http://dx.doi.org/10.1002/art.30541
29. Jing ZC, Jiang X, Han ZY, et al. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J 2009;33:1354–60. http://dx.doi.org/10.1183/09031936.00169608
30. Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369:330–40. http://dx.doi.org/10.1056/NEJMoa1209655
31. Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996;334:296–302. http://dx.doi.org/10.1056/NEJM199602013340504
32. Khanna D, Gladue H, Channick R, et al. Recommendations for screening and detection of connective tissue disease associated pulmonary arterial hypertension. Arthritis Rheum 2013;65:3194–201. http://dx.doi.org/10.1002/art.38172
33. Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006;44:1502–10. http://dx.doi.org/10.1002/hep.21431
34. Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 1996;71:543–51. http://dx.doi.org/10.1016/S0025-6196(11)64110-4
35. Valerio CJ, Schreiber BE, Handler CE, Denton CP, Coghlan JG. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013;65:1074–84. http://dx.doi.org/10.1002/art.37838
36. Hachulla E, de Groote P, Gressin V, et al. The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multicenter nationwide longitudinal study in France. Arthritis Rheum 2009;60:1831–9. http://dx.doi.org/10.1002/art.24525
37. Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62(Suppl):D13–D21. http://dx.doi.org/10.1016/j.jacc.2013.10.035
38. Grunig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009;119:1747–57. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.800938
Suggested further reading:
Lang IM, Benza R. Pulmonary hypertension: chapters of innovation and tribulation. Eur Heart J 2012;33:961-8. http://dx.doi.org/10.1093/eurheartj/ehs049
de Perrot M, Fadel E, McRae K, et al. Evaluation of persistent pulmonary hypertension after acute pulmonary embolism. Chest 2007;132:780–5. http://dx.doi.org/10.1378/chest.06-2493
Kiely DG, Elliot CA, Sabroe I, Condliffe R. Pulmonary hypertension: diagnosis and management. BMJ 2013;346:f2028. http://dx.doi.org/10.1136/bmj.f2028
close window and return to take test
All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.