Lipoproteins play a pivotal role in the development of atherosclerosis, where apolipoprotein B-containing lipoproteins are considered pro-atherogenic and high-density lipoprotein anti-atherogenic. The retention and accumulation of modified low-density lipoprotein in foam cells within the intima of the arterial vessel wall is characteristic of the atherosclerotic process. Conversely, high-density lipoprotein plays an important role in the efflux of excess free cholesterol from the arterial wall through the process of reverse cholesterol transport. High-density lipoprotein also has antioxidant and anti-inflammatory properties that may also confer a protective effect on the vasculature. Statins are the first-line treatment for lowering low-density lipoprotein, but the residual risk of disease remains high. Novel therapies are under investigation that may offer a new therapeutic approach to treating atherosclerosis and additional protection against cardiovascular disease.
Introduction
Atherosclerosis is a systemic disease of the large- and medium-sized muscular arteries, which is characterised by endothelial dysfunction, vascular inflammation, and the build up of lipids, cholesterol, calcium, and cellular debris within the intima of the vessel wall. This build up results in plaque formation, vascular remodelling, acute and chronic luminal obstruction, abnormalities of blood flow and diminished oxygen supply to target organs.1 Plaque rupture and thrombosis result in the acute clinical complications of atherosclerosis.
The process of atherosclerosis begins early in life and progresses over many decades. Rupture of a plaque or denudation of the endothelium overlying a fibrous plaque can expose the highly thrombogenic subendothelium and lipid core, which may result in thrombus formation that can partially or completely occlude the flow in the artery.1
This paper investigates the pathophysiology of atherosclerosis, the pivotal role of lipoproteins in this process and the potential of novel strategies to reduce the cardiovascular burden of atherosclerosis.
The pathophysiology of atherosclerosis
Atherosclerosis is an inflammatory disorder initiated by the subendothelial retention and accumulation of apolipoprotein B (ApoB)-containing lipoproteins such as low-density lipoprotein (LDL) in the arterial intima.2 This retained LDL is subsequently oxidised (oxLDL) and elicits a series of biological responses that lead to an inappropriate inflammatory response. Endothelial cells are stimulated to express monocyte chemotactic protein-1 (MCP-1) that, in turn, attracts monocytes into the subendothelial space. The oxLDL also promotes the differentiation of monocytes into macrophages, under the action of granulocyte-macrophage colony-stimulating factor (GM-CSF). The macrophages take up the oxLDL in a process that converts them into foam cells, the hallmark cell of atherosclerosis.3
LDLs are the main atherogenic lipoproteins in plasma, although catabolic remnants of chylomicrons and very-low-density lipoproteins (VLDLs) have been implicated in the development of atherosclerosis. The retention of ApoB lipoproteins is also amplified in established atherosclerotic plaques, further accelerating the inappropriate inflammatory response.2 In addition to taking up oxLDL, macrophages in the vessel wall also secrete cytokines, including tumour necrosis factor (TNF)-α and interleukin-1, that stimulate endothelial cells to express adhesion proteins, including vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule-1 (ICAM-1) and selectins. These adhesion proteins bind monocytes to the endothelium, making them available for recruitment into the artery wall by MCP-1. This process creates a vicious cycle that greatly amplifies the effects of the modified LDLs to promote atherogenesis.3 In addition, there are interactions between foam cells, T-lymphocytes (CD36, CD40), the vessel wall and smooth muscle cells, mediated by various cytokines including matrix metalloproteinases (MMPs) and endothelin-1 that determine the ultimate fate of the lesions.3
The role of lipoproteins in atherosclerosis
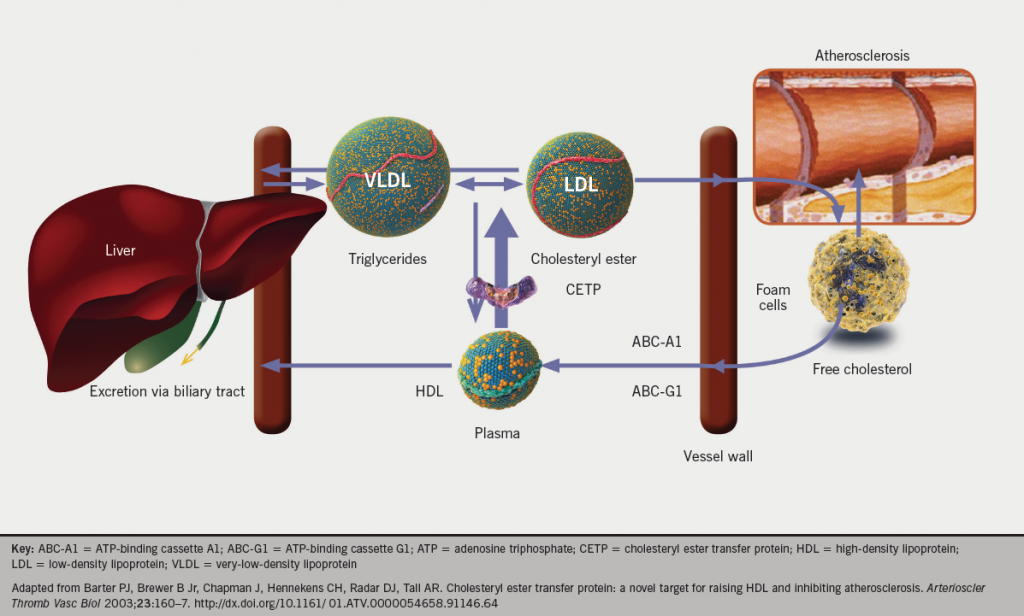
Elevated levels of LDL and other ApoB-containing lipoproteins are associated with an increased risk of cardiovascular disease. VLDL is produced by the liver, and through the actions of cholesteryl ester transfer protein (CETP) it becomes depleted of triglyceride (TG), resulting in the formation of LDL. The presence of additional risk factors, such as hypertension or smoking, increases the likelihood that pro-atherogenic particles will enter the macrophages in the vessel wall and contribute to the atherosclerotic plaque.4 In contrast, high-density lipoprotein (HDL) promotes the efflux of cholesterol from the foam cells and returns it to the liver, thus inhibiting the progression of atherosclerosis.An inverse relationship between the level of HDL and the risk of coronary heart disease (CHD) has been consistently demonstrated in population studies.4,5
Macrophages in the vessel wall can protect against cholesterol toxicity by converting free cholesterol to cholesteryl ester, or by effluxing cholesterol to extracellular acceptors – generally HDL particles.6 During reverse cholesterol transport, a proportion of the cholesteryl esters in HDL are transferred by CETP to pro-atherogenic VLDL and LDL (figure 1). Some of the cholesteryl esters in the VLDL/LDL pool can subsequently be returned to the liver via the LDL-receptor pathway, a process mediated by proprotein convertase subtilisin kexin type 9 serine protease (PCSK9);7 however, cholesteryl esters can also be delivered back to peripheral tissues. PCSK9 binds to hepatocyte LDL-receptors both intracellularly and extracellularly, leading to their lysomal degradation and, therefore, modifying the level of circulating LDL-cholesterol in the plasma.8,9 It is regulated through sterol regulatory element binding protein-2 (SREBP-2) – a transcription factor important for the regulation of genes involved in cholesterol biosynthesis as well as synthesis of the LDL-receptor itself.10
Macrophages have several pathways for efflux of cholesterol, with different HDL-associated apolipoproteins acting as acceptor molecules. Free cholesterol is effluxed to lipid-poor apolipoprotein A-I (ApoA-I) via the adenosine triphosphate (ATP)-binding cassette A1 (ABC-A1) pathway, and to mature apolipoprotein E (ApoE)-containing HDL via the ATP-binding cassette G1 (ABC-G1) pathway.11 As ABC-G1 is highly expressed in macrophages and mediates cholesterol efflux from foam cells to mature HDL, it has been suggested that this pathway is important for the atheroprotective properties of HDL.12
The composition of HDL-particles influences the amount of cholesterol they can carry. HDLs enriched with ApoE can carry larger amounts of cholesterol than those containing ApoA-I alone.13 From the lipid-poor ApoA-I particles, through HDL3 and HDL2, the action of lecithin:cholesterol acyltransferase (LCAT) esterifies cholesterol taken up by the particles, allowing it to be packaged in the lipid core as cholesteryl esters. Although the major apolipoprotein of both HDL3 and HDL2 is ApoA-I, HDL2 also contains significant amounts of ApoE. The ApoE allows HDL2 particles to further expand by LCAT-mediated enrichment of the core with cholesteryl esters. The larger HDLs with ApoE (HDL2 or HDL1) can deliver cholesterol to the liver directly, as ApoE itself is an effective ligand for the LDL-receptor.13Furthermore, the increased content of ApoE and LCAT in these particles has been shown to promote cholesterol efflux from macrophages in an ABC-G1-dependent pathway.
Beyond its role in reverse cholesterol transport, HDL may have a number of properties that, together or individually, confer a protective effect on the vasculature. These includeantioxidant properties and inhibition of adhesion molecule expression by the endothelium.14Thus, by inhibiting LDL oxidation and MCP-1 expression, HDL may help to prevent two of the key factors in the development of atherosclerosis.In addition, HDL may have anti-inflammatory effects, in part exerted through reduction of neutrophil infiltration into the injured endothelium.14HDL also stimulates the generation of nitric oxide (NO), thus reducing the endothelial dysfunction that may precede development of atherosclerosis.15,16In addition to stimulating NO production, HDL has a number of effects that may confer antithrombotic properties, including inhibition of platelet-activating factor, of thrombin, and of tissue factor.15,16Finally,in vitro studies have shown that HDL promotes endothelial cell proliferation and migration, and it also has anti-apoptotic effects on endothelial cells. Together, these properties may help to protect the endothelium against lesions that would otherwise help to promote atherogenesis.15,16
Lipoprotein(a), or Lp(a), is an analogue of LDL that has been established as a causal and independent risk factor for cardiovascular disease. It consists of an LDL-particle with a glyocoprotein known as apolipoprotein(a) (Apo[a]) attached to its associated ApoB – a process that takes place intracellularly in hepatocytes. Apo(a) has a close structural similarity to plasminogen, and, therefore, along with its LDL-type properties, Lp(a) also has antifibrinolytic properties – potentially promoting both atherosclerosis and thrombosis. Lp(a) levels are largely genetically determined and values of >500 mg/L are considered undesirably high. Its levels are generally lower in European populations and also appear to be unaltered by other cardiovascular risk factors.17
Our current understanding of the overall contributions of LDL and HDL to the atherosclerotic disease process may be summarised as follows. The pro-atherosclerotic pathway is promoted by ApoB-containing lipoproteins, especially LDL; consequently, a high concentration of ApoB-lipoproteins promotes atherogenesis, because lipids will be taken up by the blood vessel, resulting in plaque formation. Conversely, the anti-atherosclerotic pathway is promoted by HDL; consequently, a high concentration of HDL (ApoA-I-containing particles) inhibits atherogenesis, as the return of cholesterol from the plaque to the liver by reverse cholesterol transport is increased. As all ApoB-containing lipoproteins are atherogenic, the measurement of non-HDL cholesterol may, therefore, be a more logical target of therapy than simply measuring the level of LDL-cholesterol.
Current treatment
Statin therapy has been demonstrated to reduce cardiovascular events significantly in large outcomes trials of patients.18 A meta-analysis of data from over 169,000 participants in 26 randomised trials showed that all-cause mortality was reduced by 10% per 1.0 mmol/L LDL reduction (rate ratio [RR] 0.90, 95% confidence interval [CI] 0.87–0.93; p<0.0001), largely reflecting significant reductions in deaths due to CHD (RR 0.80, 99% CI 0.74–0.87; p<0.0001) and other cardiac causes (RR 0.89, 99% CI 0.81–0.98; p=0.002).18
However, while statins are a first-line treatment for lowering elevated LDL they raise HDL by 5–15% at most.19 In addition, statins have been shown to increase the expression of PCSK9, which could limit their efficacy as the dose is increased.20-23 Combination therapy with a statin plus ezetimibe, a cholesterol absorption inhibitor, may represent an alternative approach to further reducing cardiovascular events. In a meta-analysis of 27 trials (>21,000 patients), there was incremental lowering of LDL-cholesterol, non-HDL-cholesterol and triglycerides by 15%, 13% and 5%, respectively, and raising of HDL-cholesterol by 1.6%, with the combination of ezetimibe plus statin compared with statin alone.24 However, so far there are limited outcomes data to support this strategy – even in high-risk patients. Although there was a significant reduction of 17% in major atherosclerotic events (RR 0.83, 95% CI 0.74–0.94; p=0.0021) in patients with advanced renal disease in the Study of Heart and Renal Protection (SHARP) trial,25 the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial failed to show significant clinical benefit in patients with aortic stenosis.26 It is expected that the ongoing large scale phase III IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) will definitively address whether this combination is of value for high-risk patients, with completion of this study estimated later in 2014.27
A combination of low levels of HDL, high TGs and small, dense LDL-particles (known as the atherogenic lipid triad) are common in patients with diabetes. Fibrates have demonstrated efficacy in substantially decreasing TG levels, modestly increasing HDL levels by around 10–20%, and moderately reducing LDL levels.19,28 A meta-analysis investigating the effects of fibrates on cardiovascular outcomes in 18 trials, providing data for over 45,000 patients, found that fibrate therapy produced a 10% relative reduction for major cardiovascular events (95% CI 0–18; p=0.048) and a 13% relative reduction for coronary events (95% CI 7–19; p<0.0001) but no benefit on stroke (–3%, 95% CI –16–9; p=0.69).29
Niacin (nicotinic acid) is currently the most effective agent to raise HDL with increases of 15–35% reported,16 and is also one of the few drugs that significantly lowers Lp(a).17 Two recent large-scale trials investigating niacin as an add-on therapy to statins in those with pre-existing stable cardiovascular disease have shown the agent to have little benefit on clinical outcomes. Both the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) and Heart Protection Study 2 – Treatment of High-Density Lipoprotein to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trials showed the addition of niacin to statin therapy did not significantly reduce the risk of the composite end point of death from CHD, non-fatal myocardial infarction (MI), ischaemic stroke or coronary revascularisation.30,31 Following the results of HPS2-THRIVE, the combination of extended-release niacin and laropiprant has been withdrawn from use.
Potential future therapies
Advances in the understanding of the vascular biology of atherosclerosis have raised the possibility of using novel therapies to address, more directly, the issue of atherosclerosis. Several novel mechanisms are being investigated, these include raising HDL through the inhibition of CETP,32,33 further lowering of LDL through inhibition of PCSK9,34 and methods to stabilise existing atherosclerotic plaques.35
CETP inhibition
Inhibition of CETP has the potential to impact on the lipid content and concentration of all lipoprotein fractions, thereby reducing the risk of atherosclerosis. Any beneficial effect of CETP inhibition on atherosclerosis may be due to decreased cholesterol uptake and increased cholesterol efflux in macrophage foam cells and in vascular cells of atherosclerotic plaques.36 A meta-analysis of 92 studies involving 113,833 participants found that the CETP genotypes exhibiting lower CETP activity are associated with decreased coronary risk.37
Novel CETP inhibitors are in development and offer a potential new therapeutic approach for elevating HDL and reducing the risk of atherosclerosis. The development of torcetrapib, a CETP inhibitor, was terminated following the ILLUMINATE (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events) trial where torcetrapib therapy resulted in an increased risk of mortality and morbidity in patients at high cardiovascular risk.38 This appeared to signal an end to CETP inhibition as a strategy in these patients. However, it has been suggested that the increased morbidity and mortality observed in the ILLUMINATE study appear to be unrelated to CETP inhibition, and instead were the result of an off-target increase in blood pressure and small but significant increases in serum aldosterone.38,39 Early studies on other CETP inhibitors, such as dalcetrapib, anacetrapib and evacetrapib, have demonstrated positive results on lipid profiles in patients with dyslipidaemia.40-42 However, following the results of a second interim analysis it was announced that the development of dalcetrapib would be halted due to a lack of meaningful clinical efficacy in the large phase III dal-OUTCOMES trial, investigating the safety and efficacy of dalcetrapib in patients with stable CHD.43 Anacetrapib remains the most advanced molecule in clinical development, showing a reduction in Lp(a) of 36.4%, in addition to its increase in HDL of 138.1%, and the ongoing phase III REVEAL (Randomized Evaluation of the Effects of Anacetrapib Through Lipid Modification) study is examining its efficacy in the reduction of major coronary events in patients with established vascular disease.44
PCSK9 inhibition
Loss-of-function mutations of PCSK9 result in higher levels of the LDL-cholesterol receptor and lower levels of LDL-cholesterol,7 and, as such, there are a number of monoclonal antibodies currently in development that bind to PCSK9 and inhibit its function. Both alirocumab (REGN727/SAR236553) and evolcumab (AMG145) have demonstrated impressive reductions in LDL-cholesterol levels as adjuncts to statin therapy in phase II trials of patients with primary hypercholesterolaemia. When added to either 10 mg or 80 mg of atorvastatin, alirocumab resulted in a significantly greater reduction in LDL-cholesterol and in Lp(a) than that attained with 80 mg of atorvastatin alone, and resulted in substantially more patients achieving LDL-cholesterol targets of 2.6 mmol/L and 1.8 mmol/L.45 Similarly, in an efficacy analysis of pooled data from four 12-week phase II studies, evolcumab showed reductions in LDL-cholesterol of up to 59% versus placebo with an acceptable safety profile.46 This class of agents is under investigation in phase III clinical trials across a variety of patient groups with hypercholesterolaemia; however, the large scale ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment With Alirocumab) and FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) studies will determine whether this additional reduction in LDL-cholesterol translates into a meaningful reduction in cardiovascular outcomes.47,48
Lp-PLA2 inhibition
Another therapeutic approach under investigation is the inhibition of lipoprotein-associated phospholipase-A2 (Lp-PLA2) to stabilise atherosclerotic plaques in an attempt to prevent rupture, commonly resulting in major cardiovascular events. Lp-PLA2 is an important regulator of lipid metabolism and inflammation that circulates with lipoprotein particles and is carried into the arterial wall with LDL-particles during the progression of atherosclerosis. Within the vessel wall, Lp-PLA 2 releases small molecules that stimulate macrophage recruitment and evolution to foam cells, leading to plaque vulnerability.35 A number of observational and epidemiological studies have shown that elevated circulating Lp-PLA2 mass or activity (or both) predicts an increased risk for incident MI and stroke, remaining significant even after adjustment for other cardiovascular risk factors.35 Darapladib, a novel Lp-PLA2 inhibitor, is currently in clinical development and early studies have shown favourable reductions in Lp-PLA2 activity, and inflammatory markers. In November 2013, it was announced that the large phase III STABILITY (Stabilisation of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial did not meet its primary end point measure of a reduction in time to any major cardiovascular event, however, it was noted that there were greater reductions in some of the pre-defined secondary end points and patient subgroups that require further investigation. These results, and the results of a second phase III trial, SOLID-TIMI52 (Stabilization of Plaques Using Darapladib – Thrombolysis In Myocardial Infarction 52), will determine the effect of these changes on major coronary events in patients with established vascular disease.49,50
Summary
Current approaches to recognise and treat atherosclerosis have reduced the incidence of cardiovascular disease, however, considerable residual risk of disease remains. Lipoproteins play a pivotal role in the pathogenesis of atherosclerosis. The LDL pathway is considered pro-atherogenic, while HDL is anti-atherogenic. HDL has an important role in reverse cholesterol transport, increasing the efflux of free cholesterol from the arterial wall, and demonstrates other antioxidant and anti-inflammatory properties that may also confer a protective effect on the vasculature. Statins are used to good effect for the reduction of LDL and in the prevention of cardiovascular disease, but, despite their use, the residual risk of disease remains high. Novel therapies are under investigation that may offer a new therapeutic approach to treating atherosclerosis and provide additional protection against cardiovascular disease. Although surrogate markers for CETP inhibitors initially looked promising, the recent failure of dalcetrapib has undermined confidence in this approach – however, ongoing studies may still prove that the effect on these markers translates into a meaningful reduction in events. Focus has since shifted to inhibition of PCSK9 as the most promising strategy to reduce residual cardiovascular risk, and we await with interest the results of the large-scale outcomes studies into this class of agents.
Acknowledgements
Editorial and writing assistance was provided by Virgo Health Education, with financial support provided by F. Hoffman-La Roche. AB and IF retain full editorial control over the content of the article, with the sponsor reviewing it for scientific accuracy only.
Conflict of interest
The authors have received no honoraria from F. Hoffman-La Roche for authoring this review.
Editors’ note
See also the editorials by Sever and Mackay, and Wagener in this issue.
Key messages
- Atherosclerosis begins in early life and progresses over many decades
- Statins are first-line therapy for lowering elevated LDL-cholesterol
- Inhibition of PCSK9 with statin therapy results in significant reduction of LDL-cholesterol
- Agents which inhibit Lp-PLA2 may help prevent plaque rupture
References
1. Boudi FB. Coronary artery atherosclerosis. New York: Medscape emedicine; 2011. Available from: http://emedicine.medscape.com/article/153647-overview#aw2aab6b2b2 [cited 22 October 2013].
2. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis. Circulation 2007;116:1832–44. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.676890
3. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 2003;10:63–71. http://dx.doi.org/10.5551/jat.10.63
4. von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 2001;21:13–27. http://dx.doi.org/10.1161/01.ATV.21.1.13
5. Barter P. Is high-density lipoprotein the protector of the cardiovascular system? Eur Heart J 2004;6(suppl):A19–A22. http://dx.doi.org/10.1016/j.ehjsup.2004.01.005
6. Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50(suppl):S189–S194. http://dx.doi.org/10.1194/jlr.R800088-JLR200
7. Grefhorst A, McNutt MC, Lagace TA, Horton JD. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res 2008;49:1303–13. http://dx.doi.org/10.1194/jlr.M800027-JLR200
8. Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007;32:71–7. http://dx.doi.org/10.1016/j.tibs.2006.12.008
9. Poirier S, Mayer G, Poupon V et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. Biol Chem 2009;284:28856–64. http://dx.doi.org/10.1074/jbc.M109.037085
10. Mayne J, Dewpura T, Raymond A et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis 2008;7:22. http://dx.doi.org/10.1186/1476-511X-7-22
11. Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest 2006;116:3090–100. http://dx.doi.org/10.1172/JCI30163
12. Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med 2008;263:256–73. http://dx.doi.org/10.1111/j.1365-2796.2007.01898.x
13. Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest 2006;116:1226–9. http://dx.doi.org/10.1172/JCI28632
14. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res 2004;95:764–72. http://dx.doi.org/10.1161/01.RES.0000146094.59640.13
15. O’Connell BJ, Genest J Jr. High-density lipoproteins and endothelial function. Circulation 2001;104:1978–83. http://dx.doi.org/10.1161/hc3901.096667
16. Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol 2003;23:1724–31. http://dx.doi.org/10.1161/01.ATV.0000094961.74697.54
17. Thompson GR, Seed M. Lipoprotein(a): the underestimated cardiovascular risk factor. Heart 2014;100:534–5. http://dx.doi.org/10.1136/heartjnl-2013-304902
18. Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. http://dx.doi.org/10.1016/S0140-6736(10)61350-5
19. Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. http://dx.doi.org/10.1001/jama.285.19.2486
20. Careskey HE, Davis RA, Alborn WE et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res 2008;49:394–8. http://dx.doi.org/10.1194/jlr.M700437-JLR200
21. Davignon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc 2009;120:163–73.
22. Costet P, Hoffmann MM, Cariou B, Guyomarc’h Delasalle B, Konrad T, Winkler K. Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis 2010;212:246–51. http://dx.doi.org/10.1016/j.atherosclerosis.2010.05.027
23. Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res 2010;51:2714–21. http://dx.doi.org/10.1194/jlr.M008144
24. Morrone D, Weintraub WS, Toth PP et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012;223:251–61. http://dx.doi.org/10.1016/j.atherosclerosis.2012.02.016
25. Baigent C, Landray MJ, Reith C et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. http://dx.doi.org/10.1016/S0140-6736(11)60739-3
26. Rossebø AB, Pedersen TR, Boman K et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. http://dx.doi.org/10.1056/NEJMoa0804602
27. Clinicaltrials.gov. NCT00202878: IMPROVE-IT: examining outcomes in subjects with acute coronary syndrome: vytorin (ezetimibe/simvastatin) vs simvastatin (P04103 AM5). Bethesda: National Library of Medicine; 2014. Available from: http://clinicaltrials.gov/show/NCT00202878 [cited 3 February 2014].
28. Tenenbaum A, Fisman EZ, Motro M, Adler Y. Optimal management of combined dyslipidemia: what have we behind statins monotherapy? Adv Cardiol 2008;45:127–53. http://dx.doi.org/10.1159/000115192
29. Jun M, Foote C, Lv J et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010;375:1875–84. http://dx.doi.org/10.1016/S0140-6736(10)60656-3
30. Boden WE, Probstfield JL, Anderson T et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. http://dx.doi.org/10.1056/NEJMoa1107579
31. Heartwire. HPS-2 THRIVE misses primary end point: no benefit of niacin/laropiprant. New York: Medscape; 2012. Available from: http://theheart.org/article/1490635.do [cited 22 October 2013].
32. Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol 2011;8:266–77. http://dx.doi.org/10.1038/nrcardio.2010.200
33. Paras C, Hussain MM, Rosenson RS. Emerging drugs for hyperlipidemia. Expert Opin Emerg Drugs 2010;15:433–51. http://dx.doi.org/10.1517/14728214.2010.481282
34. Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther 2013;35:1082–98. http://dx.doi.org/10.1016/j.clinthera.2013.06.019
35. Corson MA. Darapladib: an emerging therapy for atherosclerosis. Ther Adv Cardiovasc Dis 2010;4:241–8. http://dx.doi.org/10.1177/1753944710375820
36. Masson D, Jiang XC, Lagrost L, Tall AR. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res 2009;50(suppl):S201–S206. http://dx.doi.org/10.1194/jlr.R800061-JLR200
37. Vasan RS, Pencina MJ, Robins SJ et al. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation 2009;120:2414–20. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.872705
38. Barter PJ, Caulfield M, Eriksson M et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22. http://dx.doi.org/10.1056/NEJMoa0706628
39. Rosenson RS. Off-target toxicity: risks associated with adrenal corticoid activation in ILLUMINATE. Curr Atheroscler Rep 2008;10:227–9. http://dx.doi.org/10.1007/s11883-008-0035-x
40. Stein EA, Stroes ES, Steiner G et al. Safety and tolerability of dalcetrapib. Am J Cardiol 2009;104:82–91. http://dx.doi.org/10.1016/j.amjcard.2009.02.061
41. Cannon CP, Shah S, Dansky HM et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406–15. http://dx.doi.org/10.1056/NEJMoa1009744
42. Nicholls SJ, Brewer HB, Kastelein JJ et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 2011;306:2099–109. http://dx.doi.org/10.1001/jama.2011.1649
43. Roche Products Limited. Roche provides update on Phase III study of dalcetrapib. Basel: Roche; 2012. Available from: http://www.roche.com/media/media_releases/med-cor-2012-05-07.htm [cited 22 October 2013].
44. Clinicaltrials.gov. NCT01252953: REVEAL: Randomized evaluation of the effects of anacetrapib through lipid modification. Bethesda: National Library of Medicine; 2011. Available from: http://clinicaltrials.gov/ct2/show/NCT01252953 [cited 22 October 2013].
45. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–900. http://dx.doi.org/10.1056/NEJMoa1201832
46. Giugliano RP, Raal F, Koren MJ et al. Safety of AMG 145, a fully human monoclonal antibody to PCSK9: data from four phase 2 studies in 1314 patients [abstract]. Eur Heart J 2013;34(suppl):135–6. http://dx.doi.org/10.1093/eurheartj/eht307.P683
47. Clinicaltrials.gov. NCT01663402: Evaluation of cardiovascular outcomes after an acute coronary syndrome during treatment with alirocumab SAR236553 (REGN727) (ODYSSEY Outcomes). Bethesda: National Library of Medicine; 2013. Available from: http://clinicaltrials.gov/show/NCT01663402 [cited 22 October 2013].
48. Clinicaltrials.gov. NCT01764633: Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER). Bethesda: National Library of Medicine; 2013. Available from: http://clinicaltrials.gov/show/NCT01764633 [cited 22 October 2013].
49. O’Donoghue ML, Braunwald E, White HD et al. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J 2011;162:613–19. http://dx.doi.org/10.1016/j.ahj.2011.07.018
50. GlaxoSmithKline. GSK announces top-line results from pivotal Phase III study of darapladib in chronic coronary heart disease. Brentford: GSK; 2013. Available from: http://www.gsk.com/media/press-releases/2013/gsk-announces-top-line-results-from-pivotal-phase-iii-study-of-d.html [cited 3 February 2014].
