Management of valve thrombosis
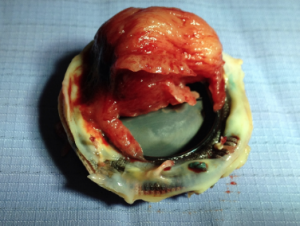
Courtesy of Mr C Blauth, Cardiothoracic Centre, Guy’s and St. Thomas’ Hospital
The risk of thrombosis is 0.1–0.3% p.a. for mechanical valves in the mitral position and 0–0.1% in the aortic position. Thrombosis presents with symptoms of obstruction (obstructive) (see figure 5) or thromboembolism (non-obstructive).
Obstructive thrombosis should be suspected in a patient with pulmonary oedema or hypotension particularly with subtherapeutic INR levels. The diagnosis is made with transthoracic echocardiography (see figure 6) while transoesophageal echocardiography (TOE) is needed to confirm the diagnosis (see figure 7) and help differentiate the other causes (pannus, vegetations).

Courtesy of Professor John Chambers
Thrombolysis can be carried out with recombinant tissue plasminogen activator (rTPa) 10 mg bolus, then 90 mg over one hour. Non-obstructive thrombosis is treated initially with heparin, unless the thrombus is ≥10 mm in diameter on TOE when redo surgery is indicated.
The management of prosthetic valve thrombosis carries a high risk. Surgery is often risky as it is performed under emergency conditions and treatment with an antifibrinolytic carries an increased risk of bleeding, systemic embolism and recurrent thrombosis.8
In general, left-sided valve thrombosis should be managed surgically while right-sided thrombosis may be managed with fibrinolytic therapy (see figures 8–10 for AHA and ESC guidelines).
Figure 8. 2014 AHA/ACC guidelines for the management of patients with valvular heart disease: Evaluation and management of suspected prosthetic valve thrombosis
This algorithm can be viewed as figure 7 in Nishimura10
Figure 9. ESC/EACTS guideline: Management of left sided obstructive prosthetic thrombosis
This algorithm can be viewed as figure 5 in Guidelines on the management of valvular heart disease (version 2012)
Figure 10. ESC/EACTS guideline: management of left sided non-obstructive prosthetic valve
This algorithm can be viewed as figure 6 in Guidelines on the management of valvular heart disease (version 2012)
Emergency surgery is recommended for all patients with:
- Thrombosed left-sided prosthetic heart valve with NYHA class III to IV symptoms
- Thrombosed left-sided prosthetic heart valve with a mobile or large thrombus (>0.8 cm2)
Fibrinolytic therapy can be considered in a patient with:
- Thrombosis of left-sided prosthetic valve, recent onset (<14 days) of NYHA class I to II symptoms and a small thrombosis10–14
- Thrombosis of right-sided prosthetic heart valves.10,16
Use of NOACs in valvular heart disease and AF17
Patients with valvular AF, defined as AF in the presence of mechanical heart valves or the presence of moderate-to-severe mitral stenosis, have been excluded from all trials with NOACS.. However patients with AF and other valvular disease (such as those with bioprothestic valves, or who have undergone valve repair) have been included in some trials. These patients do not usually require anticoagulation unless AF is present and they would therefore be a candidate for NOAC therapy.
In the large NOAC trials to date, the following sub-group analyses have been made:
- ARISTOTLE trial:18 the relative benefit of apixaban over warfarin was preserved despite a higher risk of bleeding and thromboembolic events in patients with moderate valvular heart disease including aortic stenosis and regurgitation, moderate mitral regurgitation, but excluding those with more than mild mitral stenosis or a history of valve surgery.
- RE-LY trial:19 patients with valvular disease had a higher risk of major bleeding (but not stroke), irrespective of anticoagulant treatment. Similar relative benefits of dabigatran over warfarin were seen in patients with valvular heart disease.
- ROCKET-AF trial:20 rivaroxaban showed similar efficacy findings as VKA although bleeding rates with rivaroxaban were higher than with VKA in patients with valvular disease, and the rate of systemic embolism (not stroke) was marginally higher with rivaroxaban.
- In a recent retrospective single centre cohort study, where patients with bioprosthetic valves were prescribed NOACs for AF, there were no incidence of ischaemic stroke and the incidence of minor and major bleeding were 8.2% and 5.9% respectively.21 The study population was small, however, with only 73 patients.
From the existing trial data, it may be reasonable to treat AF in patients with moderate-to-severe valvular heart disease (including aortic heart disease but excluding moderate and severe mitral valve stenosis) with NOACs, although the benefit of thromboembolism and bleeding risk have to be considered carefully for each patient (see table 3).

NOACs can also be considered in patients with bioprosthetic heart valves or after valve repair (conditions that by themselves do not require oral anticoagulation). However, in light of the RE-ALIGN findings, it is not recommended to use NOACs during the first three and six months post-operatively, since the study showed inferiority of dabigatran compared with warfarin.
It is important to highlight that the American guidelines do not recommend NOACS in patients with biological heart valves or after valve repair.
Use of NOACS in mechanical heart valves
NOACs are currently not licensed as antithrombotic prevention in mechanical heart valves. The RE-ALIGN trial22 is the largest prospective trial to date which was set up to assess the safety and efficacy of the use of dabigatran in mechanical heart valves. It is a phase 2 dose validation study where patients who had undergone previous aortic or mitral valve replacement (<7 days or >3 months) were randomised to receive warfarin or dabigatran in a 2:1 ratio. The trial was prematurely terminated after the enrolment of 252 patients because of an excess of thromboembolic and bleeding events in the dabigatran group. Ischaemic or unspecified stroke occurred in nine patients (5%) in the dabigatran group and in no patients in the warfarin group. Major bleeding occurred in seven patients (4%) and two patients (2%), respectively.
Studies with rivaroxaban have been mainly in animal or in in vitro models. Preliminary data with porcine model has been encouraging.23 Grieten et al inserted a modified bileaflet mechanical valve conduit that bypassed the native, ligated descending thoracic aorta in 30 swine. Post-operatively, the animals were randomly assigned to groups receiving no anticoagulation (n = 10), enoxaparin at 2 mg/kg subcutaneously twice daily (n = 10) or rivaroxaban at 2 mg/kg orally twice daily (n = 10). Rivaroxaban was found to be more effective than enoxaparin for short-term thromboprophylaxis of mechanical valve prosthetics in the heterotopic aortic position. It also reduced valve thrombus and platelet deposition on day 30 following implantation without increased adverse events.
Further studies are currently underway to assess the long-term safety and efficacy of NOACs in the setting of mechanical heart valve replacement.
close window and return to take test
References and suggested reading
1. Butchart EG, Li HH, Payne N, Buchan K, Grunkemeier GL. Twenty years’ experience with the Medtronic Hall valve. J Thorac Cardiovasc Surg 2001;121:1090–100. http://dx.doi.org/10.1067/mtc.2001.113754
2. Elkayam U, Bitar F. Valvular heart disease and pregnancy: part II: prosthetic valves. J Am Coll Cardiol 2005; 46:403–10. http://dx.doi.org/10.1016/j.jacc.2005.02.087
3. Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000;160:191–6. http://dx.doi.org/10.1001/archinte.160.2.191
4. Quinn J, Klemperer Von K, Brooks R, Peebles D, Walker F, Cohen H. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single-center experience. Haematologica 2009;94:1608–12. http://dx.doi.org/10.3324/haematol.2008.002840
5. McLintock C, McCowan LME, North RA. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG 2009;116:1585–92. http://dx.doi.org/10.1111/j.1471-0528.2009.02299.x
6. Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004;92:747–51. http://dx.doi.org/10.1160/th04-06-0337
7. Grunkemeier GL, Li H-H, Naftel DC, Starr A, Rahimtoola SH. Long-term performance of heart valve prostheses. Curr Probl Cardiol 2000;25:73–156. http://dx.doi.org/10.1053/cd.2000.v25.a103682
8. Roudaut R, Lafitte S, Roudaut M-F, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis 2009;102:269–77. http://dx.doi.org/10.1016/j.acvd.2009.01.007
9. Castellano JM, Narayan RL, Vaishnava P, Fuster V. Anticoagulation during pregnancy in patients with a prosthetic heart valve. Nature Reviews Cardiology 2012;9:415–24. http://dx.doi.org/10.1038/nrcardio.2012.69
10. Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary : A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2438–88. http://dx.doi.org/10.1016/j.jacc.2014.02.537
11. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease: Executive Summary : A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease). J Am Coll Cardiol 2008;52:1890–947. http://dx.doi.org/10.1016/j.jacc.2008.10.002
12. Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol 2004;43:77–84. http://dx.doi.org/10.1016/j.jacc.2003.08.028
13. Hirsh J, Dalen J, Guyatt G. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):1S-2S.
14. Roudaut R, Lafitte S, Roudaut M-F et al. Fibrinolysis of mechanical prosthetic valve thrombosis: a single-center study of 127 cases. J Am Coll Cardiol 2003;41:653–8. http://dx.doi.org/10.1016/S0735-1097(02)02872-3
15. Regitz-Zagrosek V, Lundqvist CB, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy. Euro Heart J 2011;32:3147–97. http://dx.doi.org/10.1093/eurheartj/ehr218
16. Cáceres-Lóriga FM, Pérez-López H, Morlans-Hernández K, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis 2006;21:185–90. http://dx.doi.org/10.1007/s11239-006-4969-y
17. Heidbuchel H, Verhamme P, Alings M, , et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17: 1467-507. http://dx.doi.org/10.1093/europace/euv309
18. Avezum A, Lopes RD, Schulte PJ, et al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease, clinical perspective. Circulation 2015;132:624–32. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.014807
19. Ezekowitz MD, Parise H, Nagarakanti R, et al. Comparison of dabigatran versus warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY® trial. J Am Coll Cardiol;2014;63(12_S).
20. Breithardt G, Baumgartner H, Berkowitz SD. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET-AF trial. Eur Heart J 2014;35:3377-85. http://dx.doi.org/10.1093/eurheartj/ehu305
21. Yadlapati A, Groh C, Malaisrie SC, et al. Efficacy and safety of novel oral anticoagulants in patients with bioprosthetic valves. Clin Res Cardiol 2015 ;Sep 18 (epub ahead of print). http://dx.doi.org/10.1007/s00392-015-0919-z
22. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14. http://dx.doi.org/10.1056/NEJMoa1300615
23. Greiten LE, McKellar SH, Rysavy J, Schaff HV. Effectiveness of rivaroxaban for thromboprophylaxis of prosthetic heart valves in a porcine heterotopic valve model. Eur J Cardiothorac Surg 2014;45:914–9. http://dx.doi.org/10.1093/ejcts/ezt545
close window and return to take test
All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.
