News from the world of cardiology.
Highlights of this year’s European Society of Cardiology Congress, held in Stockholm, Sweden, from August 28th to September 1st included a new drug which benefits heart failure by slowing heart rate, and more exciting results from oral compounds that could replace warfarin in various indications.
 Highlights of this year’s European Society of Cardiology Congress, held in Stockholm, Sweden, from August 28th to September 1st included a new drug which benefits heart failure by slowing heart rate, and more exciting results from oral compounds that could replace warfarin in various indications.
Highlights of this year’s European Society of Cardiology Congress, held in Stockholm, Sweden, from August 28th to September 1st included a new drug which benefits heart failure by slowing heart rate, and more exciting results from oral compounds that could replace warfarin in various indications.
SHIFT: ivabradine shows benefit in heart failure
A new agent that slows heart rate by inhibiting sodium-potassium channels found in the sino-atrial node of the heart appears to benefit heart failure patients when added on to current therapy.
The agent, ivabradine, was associated with a significant 18% drop in the composite rate of cardiovascular death or heart-failure hospitalisation, compared with placebo, in the SHIFT trial (table 1). It was also associated with a reduction of 26% in heart-failure events leading to hospitalisation or death.
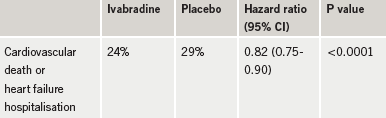
SHIFT (Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial) involved 6,500 patients with New York Heart Association (NYHA) class II – IV heart failure, left ventricular ejection fraction of 35% or less, and a prior hospitalisation for worsening heart failure within the previous 12 months. They were randomised to either ivabradine or placebo. All patients were receiving currently recommended heart failure medication, and had a heart rate of at least 70 bpm.
Principal investigator Professor Michel Komajda (Pitié Salpetrière Hospital, Paris, France) said: “These results have been achieved in addition to the effects of other medications. Heart failure and high heart rates are extremely common so it is very good news for patients and doctors that, even when using the best current drug treatment available, ivabradine further reduces the risk of death or hospitalisation by over 25%. Ivabradine has only one known cardiac action, so this opens a fascinating area of research. The SHIFT trial has demonstrated, for the first time, that reducing heart rate alone is beneficial for patients with heart failure.”
The main side-effect of ivabradine is bradycardia, which occurred in 10% of patients in this study, leading to withdrawal in 1% of patients receiving the drug.
The main concern voiced about the SHIFT trial was that patients might not have been on optimal doses of beta blockers and the benefits brought about by ivabradine may also have been seen by raising the beta-blocker dose. But designated discussant of they trial, Dr Inder Anand (University of Minnesota, USA), dismissed these concerns, noting that higher doses of beta blockers have not been shown to reduce heart rate further and, in the real world, physicians are unable to increase doses of beta blockers to target levels because of concerns about side effects.
FUTURA OASIS 8: use standard dose heparin with fondaparinux in PCI
Acute coronary syndrome (ACS) patients being treated with fondaparinux can be given a standard dose of heparin during percutaneous coronary intervention (PCI) to reduce the risk of catheter thrombosis without increasing risk of major bleeding, according to the results of FUTURA OASIS 8 (Fondaparinux Trial with UFH during Revascularisation in Acute Coronary Syndromes).
An earlier trial, OASIS 5, had shown that anticoagulation with fondaparinux was more effective than enoxaparin in reducing mortality and serious bleeding rates in ACS patients, but rates of catheter thrombosis during angioplasty with fondaparinux were found to be higher than with enoxaparin, which prompted the adjunctive use of unfractionated heparin to prevent clotting in patients treated with fondaparinux. But there has been uncertainty about the optimal dose of heparin to use.
FUTURA OASIS 8 trial randomised 2,026 patients receiving fondaparinux 2.5 mg daily to low fixed dose heparin (50 U/kg) or standard dose heparin (85 U/kg or 60 U/kg with GP IIb/IIIa inhibitors) at the time of PCI.
Results showed no significant difference in the primary outcome of peri-PCI major bleeding/minor bleeding/major vascular complications. Major bleeding rates were 1.4% in those given low-dose heparin and 1.2% the standard dose. Minor bleeding was reduced in the low-dose group, but this was accompanied by a trend towards higher risk of death, myocardial infarction or target vessel revascularisation. The rates of catheter thrombosis were very low in both groups (0.5% and 0.1% in the low and standard dose, respectively).
It was also noted that the rates of major bleeding in FUTURA-OASIS 8 were not significantly different from that observed in the fondaparinux alone arm of the OASIS 5 trial (1.5%) but lower than in the enoxaparin arm (3.6%).
AVERROES: new factor Xa inhibitor prevents stroke in AF patients
Another breakthrough in anticoagulation therapy appears to be on the horizon with the new oral factor Xa inhibitor, apixaban, having shown a clinically important reduction in stroke and systolic embolism compared with aspirin in high-risk atrial fibrillation (AF) patients unsuitable for treatment with warfarin in the AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke) trial.
The 5,600-patient trial was terminated earlier this year following an interim analysis showing a reduced rate of stroke/systolic embolism in apixaban-treated patients compared with those on aspirin, without a significant increase in major bleeding (table 1).
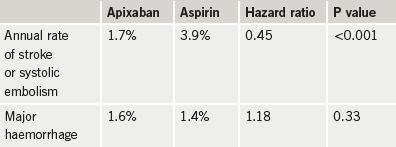
The rate of haemorrhagic stroke was 0.2% per year in both treatment groups and there was no evidence of hepatic toxicity or other major adverse events.
Principal investigator, Dr Stuart Connolly (McMaster University, Hamilton, Ontario, Canada), described the results of the trial as “truly impressive”. He added: “The reduction in stroke and systemic embolism is very important and the increased risk of haemorrhage is small. It appears that apixaban will be an excellent treatment for the many patients with atrial fibrillation who are unsuitable for warfarin. These findings will reduce the burden of stroke in society.”
Apixaban has already been investigated for the prevention of deep vein thrombosis, following orthopaedic surgery, and is also being studied against warfarin in AF patients suitable for warfarin.
RESPONSE: nurse-led programme improves risk factors in ACS patients
A six-month out-patient prevention programme conducted by nurses resulted in sustained improvements in the control of cardiovascular risk factors, including cholesterol levels and blood pressure, in acute coronary syndrome (ACS) patients.
The nurses were able to increase the proportion of patients with good control of risk factors by 40% (defined as at least seven out of nine risk factors on target) and to reduce the calculated risk of dying in the next 10 years by about 17%.
Principal investigator of the RESPONSE (Randomised Evaluation of Secondary Prevention by Outpatient Nurse Specialists) trial, Professor Ron Peters (Academic Medical Center, Amsterdam, The Netherlands) explained that the preventive aspect of treatment is given insufficient priority and that new approaches are needed to realise the full benefits of prevention in patients who have suffered a cardiac event.
The trial evaluated the effect of a nursing programme in 754 patients hospitalised for an acute coronary complication. They were randomised to either usual care alone or usual care plus a six-month nursing intervention that included four extra visits to the out-patient clinic for advice on healthy lifestyle and monitoring of major risk factors.
“The nurse programme was practical and well attended by the patients,” Professor Peters said. “More than 93% of patients attended all visits to the nurse. These findings are very encouraging and support the initiation of prevention programmes by nurses to help patients reduce their risk of future complications.”
Genetic profiling targets benefits of ACE inhibitors
The beneficial effects of angiotensin converting enzyme (ACE) inhibitors could be increased by targeting the therapy to those patients most likely to benefit, according to a new genetic study.
Presenting the study, Dr Jasper Brugts (Erasmus University Medical Centre, Rotterdam, The Netherlands) explained that his team analysed 12 candidate genes that were determined as being within the pharmacodynamic pathway of ACE inhibitors, taking in 52 single nucleotide polymorphisms (SNPs), in patients taking part in the EUROPA (European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease) trial of the ACE inhibitor, perindopril.
Three SNPs, located in the angiotensin-II type I receptor genes and bradykinin type I receptor genes, were significantly associated with the treatment benefit of perindopril after multivariate adjustment for confounders and correction for multiple testing. A pronounced treatment benefit was observed in a subgroup of 73.5% of the patients, while no benefit was apparent in the remaining 26.5%. An interaction effect of similar direction and magnitude, although not statistically significant, was observed in a preliminary confirmatory analysis of over 1,000 patients with cerebrovascular disease, who were treated with perindopril or placebo from the PROGRESS-trial, Dr Brugts reported.
This research study is the first to identify genetic determinants of the treatment benefit of ACE inhibitor therapy. “If confirmed in subsequent studies, our findings open the route to individualise therapy by pharmacogenetic profiling. Such individualised therapy could revolutionise medical drug therapy by prescribing drugs only to those patients most likely to benefit from the therapy. This would not only increase efficacy, but also decrease unnecessary treatment of patients and avoid unwanted side effects, thereby decreasing the overall costs,” Dr Brugts added.
No benefit of omega-3 fatty acids in patients with previous MI
Low doses of omega-3 fatty acids did not reduce the overall rate of major cardiovascular events in patients who have had a previous myocardial infarction (MI) in the Alpha Omega Trial.
Presenting the trial, Professor Daan Kromhout (Wageningen University, the Netherlands) explained that n-3 (or omega-3) fatty acids can be divided in two main classes: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) derived from fish; and alpha-linolenic acid (ALA) from plant foods such as soybean oil and walnuts. He noted that several intervention studies in cardiac patients have shown that a daily intake of 1-2 g of EPA + DHA via fish oil capsules has been associated with a reduced mortality from coronary heart disease, and epidemiological studies in healthy populations have also suggested that 250 mg EPA + DHA or eating fish once or twice a week can lower the risk of cardiovascular disease. For ALA, there is less evidence of a cardioprotective effect.
The Alpha Omega Trial was designed as a dietary intervention study to examine the effect of low doses of omega-3 fatty acids on major cardiovascular events. In the study, 4,837 men and women who had suffered an MI approximately four years previously were randomised to daily use of one of four margarines for 40 months: containing EPA + DHA (400 mg/day); ALA (2 g/day); both EPA + DHA and ALA; or placebo.
Results showed no reduction in major cardiovascular events in any of the omega-3 groups compared to placebo. However, among sub-groups, there was a borderline significant reduction in major cardiovascular events in women who received ALA. In addition, in patients with diabetes, omega-3 fatty acids were protective against ventricular arrhythmia-related events, and the EPA + DHA combination appeared to be associated with a reduction in CHD mortality.
Professor Kromhout suggested that the lack of any effect in the overall population may have been due to the amount of other treatments being taken by the patients, with 98% on antithrombotic agents, 90% on antihypertensive drugs, and 86% on lipid lowering drugs. “We found that cardiovascular mortality rate in the study population was only half that expected, probably because of their excellent treatment. This may also be why the rate of major cardiovascular events during follow-up was no lower in the fatty acid groups than in the placebo group,” he speculated.
ISAR-REACT 3A: lower doses of heparin best for PCI
Lower doses of unfractionated heparin are preferable to higher doses in percutaneous coronary intervention (PCI), the results of the ISAR-REACT 3A trial suggest.
Presenting the trial, Dr Stefanie Schulz (Deutsches Herzzentrum, Munich, Germany) explained that although unfractionated heparin has been the standard anti-thrombotic agent in interventional cardiology for decades, there is still no solid evidence from large clinical trials to guide its dosing during PCI.
The current study examined a contemporary heparin dose of 100 U/kg in 2,505 patients undergoing elective PCI, and compared outcomes with a historical control group of patients receiving heparin at a dose of 140 U/kg in the previously conducted ISAR-REACT-3 trial.
Results at 30 days showed that the primary net clinical outcome (a composite of death, myocardial infarction/ urgent target vessel revascularisation and bleeding) was significantly reduced with the lower heparin dose (7.3% vs. 8.7%, P=0.045). The 100 U/kg heparin dose also met the criterion of non-inferiority compared to the bivalirudin arm of ISAR-REACT 3.
But discussant of the trial, Dr Christian Hamm (Kerckhoff-Klinik, Bad Nauheim, Germany), pointed out that 100 U/kg was actually now thought of as a relatively high dose of heparin, and that lower doses needed to be tested.
ANTIPAF: no effect of angiotensin blocker on paroxysmal AF
The ANTIPAF (ANgiotensin II anTagonists In Paroxysmal Atrial Fibrillation) trial has put an end to speculation that angiotensin II receptor blockers could reduce the recurrence of atrial fibrillation (AF), showing no such effect of olmesartan in this indication.
The trial included 425 patients with documented episodes of paroxysmal AF who were stratified according to presence of beta-blocker therapy and randomised to placebo or olmesartan (40 mg/day). Patients were followed using daily trans-telephonic ECG recordings independent of symptoms and were encouraged to submit further tele-ECGs in any case of AF-related symptoms.
Results showed that the primary end point of the trial – the percentage of days with documented episodes of paroxysmal AF throughout 12 months of follow-up – showed no significant difference in the two groups. However, the time to prescription of recovery medication (amiodarone) was longer in patients treated with olmesartan than in those receiving placebo
Discussing the trial, Professor John Camm (St George’s Hospital, London) noted that angiotensin receptor blockers were effective in primary prevention of AF, but this trial adds to other recent evidence that they don’t seem to help in patients with persistent AF.
Take care when switching to generic statins
The switching of patients from branded to generic statins is having an adverse effect on lipid level for many patients, according to a Dutch study.
The study was undertaken to determine dose-specific patterns associated with switching patients in the Netherlands from the Lipitor-branded atorvastatin to generic simvastatin. Researchers took a representative sample of pharmacist dispensing data from across the Netherlands, and combined this information with published data on the dose-specific effects of each drug. “Our research demonstrated that many patients were, in fact, receiving a non-equivalent dose after switching to the generic drug,” reported Professor Danny Liew (University of Melbourne).
Results showed that in the first three months of 2009, over one third of patients who had initially been prescribed atorvastatin (Lipitor®) had been switched to a less potent dose of simvastatin. “The predicted net effect of this would be at least a 5 to 6% increase in LDL (low-density lipoprotein), which translates to a 3% average increase in the risk of heart disease and stroke,” Professor Liew added.
Rivaroxaban looks good in DVT treatment
Another promising new oral anticoagulant showed good results in the treatment of deep vein thrombosis (DVT). Rivaroxaban showed similar rates of efficacy and bleeding compared with standard treatment (enoxaparin followed by a vitamin K antagonist such as warfarin) in the treatment of patients with acute, symptomatic DVT in the EINSTEIN DVT study.
The trial involved 3,400 patients. The primary end point of recurrent symptomatic venous thromboembolism (ie, the composite of recurrent DVT, non-fatal or fatal pulmonary embolism) occurred in 2.1% of the rivaroxaban recipients and 3.0% of the subjects receiving standard therapy. The primary safety end point – major and clinically relevant non-major bleeding – occurred in 8.1% of both groups.
Principal investigator, Professor Harry Büller (Academic Medical Center, Amsterdam, The Netherlands) said: “The single-drug approach with rivaroxaban will provide clinicians and patients with an attractive, simple, alternative regimen for the initial and long-term treatment of deep vein thrombosis.”
PLATO and CURE genotype results – when is genetic testing relevant in ACS patients?
New genotype analysis results from the PLATO (A Study of Platelet Inhibition and Patient Outcomes) and TRITON-TIMI 38 studies together suggest that the antiplatelet agents ticagrelor and prasugrel are not affected by variations in the CYP2C19 gene, which can affect clopidogrel.
However, similar results from the PLATO and CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trials in patients taking clopidogrel suggest that the “loss of function” CYP2C19 alleles actually do not appear to be a problem in terms of outcomes during chronic treatment. If genetic testing with clopidogrel is necessary, it only seems relevant during the first month of treatment or perhaps if a stent is placed.
The genetic results from the PLATO and CURE trials were presented at the ESC meeting. The PLATO results were simultaneously published along with those from the TRITON study in The Lancet (DOI:10.1016/S0140-6736(10)61273-1 and 61274-3), and the genetic results from CURE were published in the New England Journal of Medicine (DOI: 10.1056/NEJMoal008410).
While the CYP2C19 loss of function alleles showed no effects on outcomes with ticagrelor or prasugrel in PLATO and TRITON-TIMI 38, respectively, results from the clopidogrel group of the PLATO trial, showed a higher event rate in patients with any of loss-of-function allele early on in the study (first 30 days) in association with the index event and often in association with an early PCI procedure. But later, after 30 days, there was no difference in event rates between those with and without loss-of-function alleles taking clopidogrel.
And in the CURE genetic study, there was no difference in outcomes in patients taking clopidogrel long-term in carriers of the loss-of-function alleles versus non-carriers. Presenting the data, Dr Guillaume Paré (McMaster University, Hamilton, Canada), pointed out that these results contrast with several previous studies in ACS patients, but they suggest that this may be due to the low number of patients receiving stenting in the CURE trial, 14.5%, compared with around 70% of previous studies looking at this issue, adding that it has consistently been shown that the greatest benefit of clopidogrel is the reduction of stent thrombosis.
Two cups of coffee a day may benefit cardiovascular health
 Moderate consumption of coffee by hypertensive elderly individuals can lead to improvements in aortic distensibility, a measure of the elasticity of arteries which is recognised as an indicator of atherosclerosis and a predictor of future cardiovascular events, new results suggest.
Moderate consumption of coffee by hypertensive elderly individuals can lead to improvements in aortic distensibility, a measure of the elasticity of arteries which is recognised as an indicator of atherosclerosis and a predictor of future cardiovascular events, new results suggest.
For the study, a team from the University of Athens conducted a health and nutrition survey using a target group of 343 men and 330 women aged between 65 and 100 – all of whom were long-term residents of the Aegean island of Ikaria. The island was selected because of its population’s high life expectancy, with an above-average proportion of residents over 90 years of age.
Coffee consumption was particularly measured during the initial phase of the study because it is a deeply embedded social tradition within the Greek population, and also because of conflicting evidence of its impact on cardiovascular health.
Lead researcher, Dr Christina Chrysohoou, explained that the pressor response to caffeine seems to be more pronounced in hypertensive or hypertension-prone subjects than in normotensive ones. Therefore, they focused on a sub-group of 235 hypertensive subjects, in whom they measured the impact of daily coffee consumption using echocardiographic indices of aortic distensibility.
Results showed that moderate coffee consumption (between one and two cups per day) is associated with higher values of aortic distensibility. Adjustments were made for various factors such as age, gender, physical activity status, creatinine levels, BMI and diabetes mellitus. There was also evidence that moderate coffee consumption leads to reduced cardiovascular disease, lower prevalence of diabetes and hyperlipidaemia, lower body mass index, better renal functions and higher creatinine clearance levels. There was no evidence, however, that increasing coffee consumption to three to five cups per day would lead to further improvements in aortic distensibility.
The researchers attribute these beneficial effects to the polyphenolic compounds found in coffee, especially traditional Greek blends, and other micronutrients, including flavonoids, magnesium, potassium, niacin and vitamin E.
ATOLL: enoxaparin for primary PCI
The low molecular weight heparin, enoxaparin, may be preferable to unfractionated heparin (UFH) in myocardial infarction (MI) patients undergoing primary percutaneous coronary intervention (PCI), the ATOLL trial suggests.
Professor Gilles Montalescot (Pitié-Salpêtrière University Hospital, Paris, France) explained that there was already good data supporting enoxaparin in elective PCI but, so far, primary PCI for ST-elevation myocardial infarction (STEMI) has traditionally been supported by unfractionated heparin.
In the ATOLL study, 910 MI patients were randomised to receive IV enoxaparin (0.5 mg/kg) or IV UFH (50-70 IU/kg with GP IIb/IIIa inhibitors, 70-100IU without GP IIb/IIIa inhibitors then adjusted according to clotting times) before coronary angiography. Primary PCI was performed through a radial access in 68% of cases, with 75% of patients receiving GP IIb/IIIa inhibitors and two-thirds of patients receiving high-dose clopidogrel.
Results (table 1) tended to favour enoxaparin, although the primary end point – death, complications of MI, procedure failure or non-CABG major bleeding at 30 days – was not significant. The main secondary end point – death, recurrent MI/acute coronary syndrome or urgent revascularisation – was significant, as was net clinical benefit (death, complication of MI or major bleeding).
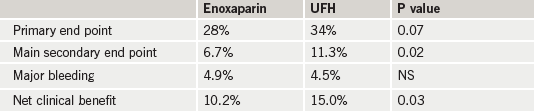
Commenting on the results, Professor Montalescot said: “Enoxaparin showed a good safety profile with a superior net clinical benefit. Our data demonstrate that this strategy, which is easier to use, is also more effective at reducing the most serious ischaemic complications of STEMI treated with primary PCI.”
New ESC guidelines announced
During the meeting, The European Society of Cardiology ESC) also announced several new sets of clinical guidelines.
Myocardial revascularisation
This guidelines included surgery, stent implantation and drug therapies. These guidelines introduce the concept of Heart Teams, essentially a grouping from across disciplines ensuring – when practical – that the patient is fully informed and takes part in the key decisions. The heart team should include one of each of the following specialists; interventional cardiologist, clinical cardiologist, and cardiac surgeon.
Atrial fibrillation
These are the first guidelines on atrial fibrillation prepared solely by the ESC, with earlier guidelines on this topic having been a collaboration with the American Heart Association and the American College of Cardiology, Divergence in practice, drug treatments and the regulatory environment compared with the US have now made it vital to create a European-specific version. The guidelines reflect notable developments in many of the conventional treatments for the condition as well as the very latest techniques to manage it.
Grown-up Congenital Heart Disease (GUCH)
This is now estimated to affect more than two million adults in Europe.
Device therapy in heart failure
In addition, the clinical practice guidelines covering device therapy in heart failure have been updated, reflecting the pace of research in this field and the importance of recently published evidence. The changes made in the guidelines take account of: recently published evidence from clinical trials, new developments in device technology and performance and more extensive understanding of treatment options and responses.
All the new guidelines can be found at: http://www.escardio.org/guidelines-surveys/esc-guidelines.
